Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
Fig. 1
Images of patient 1 who underwent cataract surgery (phacoemulsification and posterior chamber intraocular lens implant) with retrobulbar anesthesia. (A) Fundus photograph showing a cherry-red spot. (B) Fluorescein angiogram showing decreased choroidal perfusion and delayed filling of the retinal artery and vein. (C) Internal carotid angiogram showing stenosis of the right ophthalmic artery (arrow) and a tortuous proximal ophthalmic artery.

Fig. 2
Images of patient 2 who underwent vitrectomy, endolaser, and intravitreal air injection for proliferative diabetic retinopathy vitreous hemorrhage. (A) Fundus photograph obtained 3 days after intraarterial thrombolysis. A cherry-red spot, retinal edema, and multiple retinal hemorrhages are apparent. (B) Spectral domain optical coherence tomography image obtained 3 days after intra-arterial thrombolysis. Increased reflectivity and inner retinal thickness (including the central macula), along with decreased outer retina reflectivity, are apparent. (C) Internal carotid angiogram showing severe stenosis of the right cervical internal carotid artery (arrow) and the ophthalmic artery.

Fig. 3
Images of patient 3, who presented with severe visual decline resulting in no light perception after vitrectomy with retrobulbar anesthesia for proliferative diabetic retinopathy and vitreous hemorrhage. All images were taken 7 days after surgery. (A) A cherry-red spot and severe arterial narrowing and sclerosis are observed in the fundus photograph. (B) Early-phase (2 : 11) fluorescein angiogram showing poor arterial filling. (C) Increased reflectivity and thickness of the inner retina are apparent in spectral domain optical coherence tomography. (D) Standard electroretinogram (ERG) in the left eye showing decreases in both scotopic and photopic wave amplitude.
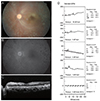
Fig. 4
Images of patient 4, who underwent vitrectomy under retrobulbar anesthesia for treatment of epiretinal membrane. Preoperative best-corrected visual acuity was 20 / 32 in the right eye. One day following surgery, best-corrected visual acuity was at the level of "hand motion" in his right eye. (A) Fundus photograph 1 day after surgery, showing a cherry red spot and marked inner retinal edema in the macula. (B) Early-phase (20 seconds after fluorescein injection) fundus fluorescein angiography 13 days after surgery, showing a mild delay in arteriovenous transit time and multiple patchy capillary nonperfusion in the posterior pole. (C) Goldmann visual field test obtained 5 months after surgery, indicating the presence of a central scotoma.

Fig. 5
Images of patient 5, who underwent cataract surgery (phacoemulsification and posterior chamber intraocular lens implant) under retrobulbar anesthesia and presented with visual decline 1 day after surgery. (A) Fundus photograph 1 day after surgery, showing a typical cherry red spot in the right eye. (B) Increased retinal reflectivity and internal retinal thickness are apparent in spectral domain optical coherence tomography. (C) Transfemoral cerebral angiogram performed 1 day after surgery, showing no definitive occlusion in the ophthalmic artery (arrow) or cerebral arteries.

Table 1
Demographic and clinical characteristics of patients with CRAO following intraocular surgery

CRAO = central retinal artery occlusion; BCVA = best-corrected visual acuity; IOP = intraocular pressure; HTN = hypertension; PE & PCL = phacoemulsification and posterior chamber intraocular lens implantation; IAT = intraarterial thrombolysis; NLP = no light perception; DM = diabetes mellitus; ICA = internal carotid artery; MI = myocardial infarction; LP = light perception; ESRD = end stage renal disease; Mx = medication; HM = handmotion; FC = finger count.
Table 2
Summary of previous reports of central retinal artery occlusion after retrobulbar anesthesia (total of 17 cases)

VA = visual acuity; BCVA = best-corrected visual acuity; FU = follow-up duration; DM = diabetes mellitus; PDR = proliferative diabetic retinopathy; FC = finger count; SCR = sickle-cell retinopathy; NA = not available; HM = hand motion; OIS = ocular ischemic syndrome; AC = anterior chamber; PE & PCL = phacoemulsification and posterior chamber intraocular lens implantation; IOP = intraocular pressure; Mx = medication; CRVO = central retinal vein occlusion; IOL = intraocular lens; NLP = no light perception; LP = light perception; HTN = hypertension.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download