Abstract
Purpose
To identify the risk factors associated with fluoroquinolone resistance in patients undergoing cataract surgery.
Methods
A total of 1,125 patients (1,125 eyes) who underwent cataract surgery at Veterans Health Service Medical Center from May 2011 to July 2012 were enrolled in this study. Conjunctival cultures were obtained from the patients on the day of surgery before instillation of any ophthalmic solutions. The medical records of patients with positive coagulase negative staphylococcus (CNS) and Staphylococcus aureus (S. aureus) cultures were reviewed to determine factors associated with fluoroquinolone resistance.
Results
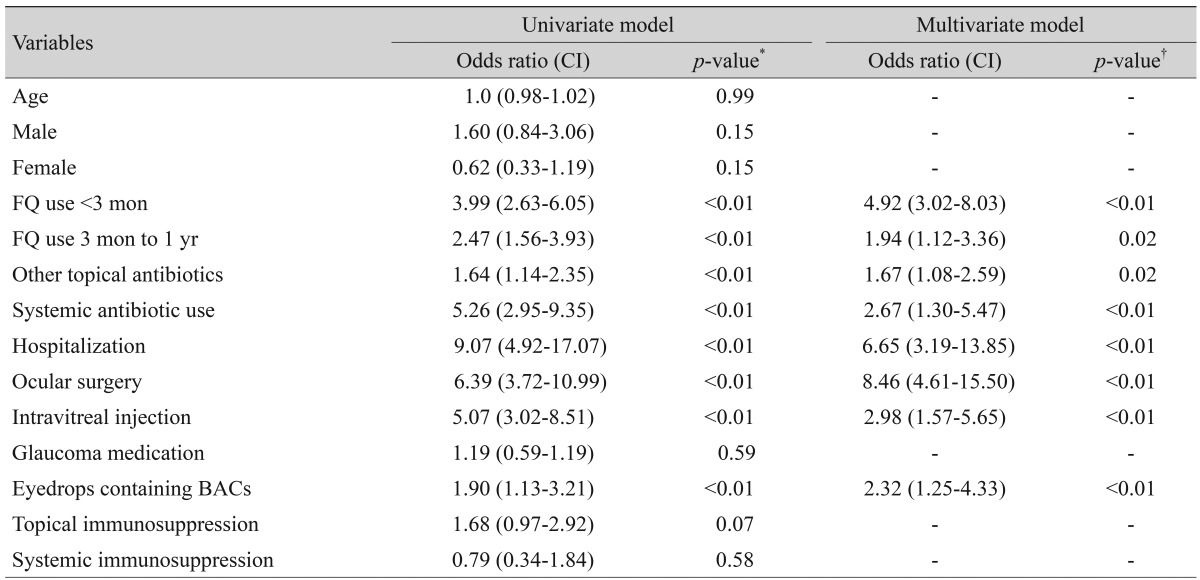
Of 734 CNS and S. aureus cultures, 175 (23.8%) were resistant to ciprofloxacin, levofloxacin, gatifloxacin, or moxifloxacin. Use of fluoroquinolone within 3 months and within 1 year before surgery, topical antibiotic use other than fluoroquinolone, systemic antibiotic use, recent hospitalization, ocular surgery, intravitreal injection and use of eyedrops containing benzalkonium chloride were significantly more frequent in resistant isolates than in susceptible isolates. In multivariable logistic regression analysis, ocular surgery (odds ratio [OR], 8.457), recent hospitalization (OR, 6.646) and use of fluoroquinolone within 3 months before surgery (OR, 4.918) were significant predictors of fluoroquinolone resistance, along with intravitreal injection (OR, 2.976), systemic antibiotic use (OR, 2.665), use of eyedrops containing benzalkonium chloride (OR, 2.323), use of fluoroquinolone within 1 year before surgery (OR, 1.943) and topical antibiotic use other than fluoroquinolone (OR, 1.673).
One of the most commonly performed surgical procedures in ophthalmology is cataract extraction and the number of these surgeries will continue to grow as the population ages. Although rare, postsurgical endophthalmitis is a dreaded complication that can be catastrophic. However, the pathophysiology of endophthalmitis and the effective preventive measures are not precisely known yet [1].
Evidence suggests that the source of pathogens causing endophthalmitis is nearly always the patient's native ocular surface and adnexal f lora [2], and therefore, reducing bacterial flora in the ocular surface before the surgical procedure is key to preventing endophthalmitis. Use of 5% povidone iodine prior to surgery is an almost universally accepted way to reduce the incidence of endophthalmitis [3,4]. Perioperative empiric topical antibiotic prophylaxis is also shown to be effective in preventing postsurgical endophthalmitis [5]. However, patterns of bacterial flora and their antibiotic susceptibility vary from region to region as well as over time [6,7]. Therefore periodic surveillance of ocular flora and antibiotic susceptibility is advocated for successful empiric perioperative antibiotic prophylaxis [2].
Topical fluoroquinolones are the most commonly used prophylactic antibiotics in cataract surgery [8]. However, the emergence of microbial resistance is a serious concern and the factors that accelerate resistance are crucial in selecting perioperative prophylactic antibiotics in cataract surgery. Herein, we report patterns of ocular flora and factors associated with fluoriquinolone resistance in ocular isolates from patients who have undergone cataract surgery.
This study was performed in accordance with the Helsinki Declaration of 1975 and its 1983 revision. This retrospective, randomized, observational study was performed at a single center. The study was approved by the institutional review board at Veterans Health Service Medical Center in Seoul, Korea. All the patients provided informed consent after receiving a full explanation of the treatment process, the risks involved and the alternatives available.
A total of 1,125 patients (1,125 eyes) who had undergone cataract surgery at Veterans Health Service Medical Center from May 2011 to July 2012 were enrolled in this study. Preoperative conjunctival cultures were obtained on the day of surgery before application of topical antibiotics (gatifloxacin 0.3%, Gatiflo; Handok, Seoul, Korea), anesthetics (proparacaine hydrochloride 0.5%, Alcaine; Alcon Laboratories, Fort Worth, TX, USA) and povidone iodine. The patient was asked to look up and both the inferior cul de sac and inferior punctum were swabbed using a sterile cotton tip without touching the eyelid margin.
Bacterial cultures were performed and disk diffusion susceptibility testing using clinical and laboratory standard institute break point was performed for multiple antibiotics including ciprofloxacin, levofloxacin, gatifloxacin, and moxifloxacin. Staphylococcus aureus (S. aureus) and coagulase negative staphylococcus (CNS) isolates were also classified as methicillin-susceptible or methicillin-resistant based on oxacillin susceptiblilty, using clinical and laboratory standard institute-defined break points.
The medical records of the patients with positive CNS and S. aureus cultures were reviewed to determine the risk factors for topical fluoroquinolone resistance. The fluoroquinolone resistance group was defined as showing resistance to any one of following tested fluoroquinolones: ciprofloxacin, levofloxacin, gatifloxacin and moxifloxacin. The following explanatory variables were investigated: age, sex, glaucoma medication, topical fluoroquinolone use within 3 months prior to surgery and between 3 months to 1 year, other topical antibiotic use, use of eyedrops containing benzalkonium chlorides (BACs), systemic antibiotic use, history of ocular surgery, hospitalization or nursing home admission, intravitreal injection including bevacizumab (Avastin; Genentech, South San Francisco, CA, USA), ranibizumab (Lucentis, Genentech) and triamcinolone, topical immunosuppression (use of topical corticosteroids) and systemic immunosuppression within 1 year prior to the bacterial culture. Systemic immunosuppression was defined as IgE deficiency, human immunodeficiency virus seropositivity, or systemic immunosuppressive therapy with agents such as oral prednisone, immunosuppressive agents, or systemic chemotherapy.
Statistical analysis of the data was performed using PASW Statistics ver. 18 (SPSS Inc., Chicago, IL, USA). The Fisher exact test was used to compare the proportions of patients in the fluoroquinolone-susceptible and fluoroquinolone-resistant groups. And the univariate and multivariate backward stepwise logistic regression analysis was used to determine risk factors for fluoroquinolone resistance. A p-value <0.05 was considered to be statistically significant.
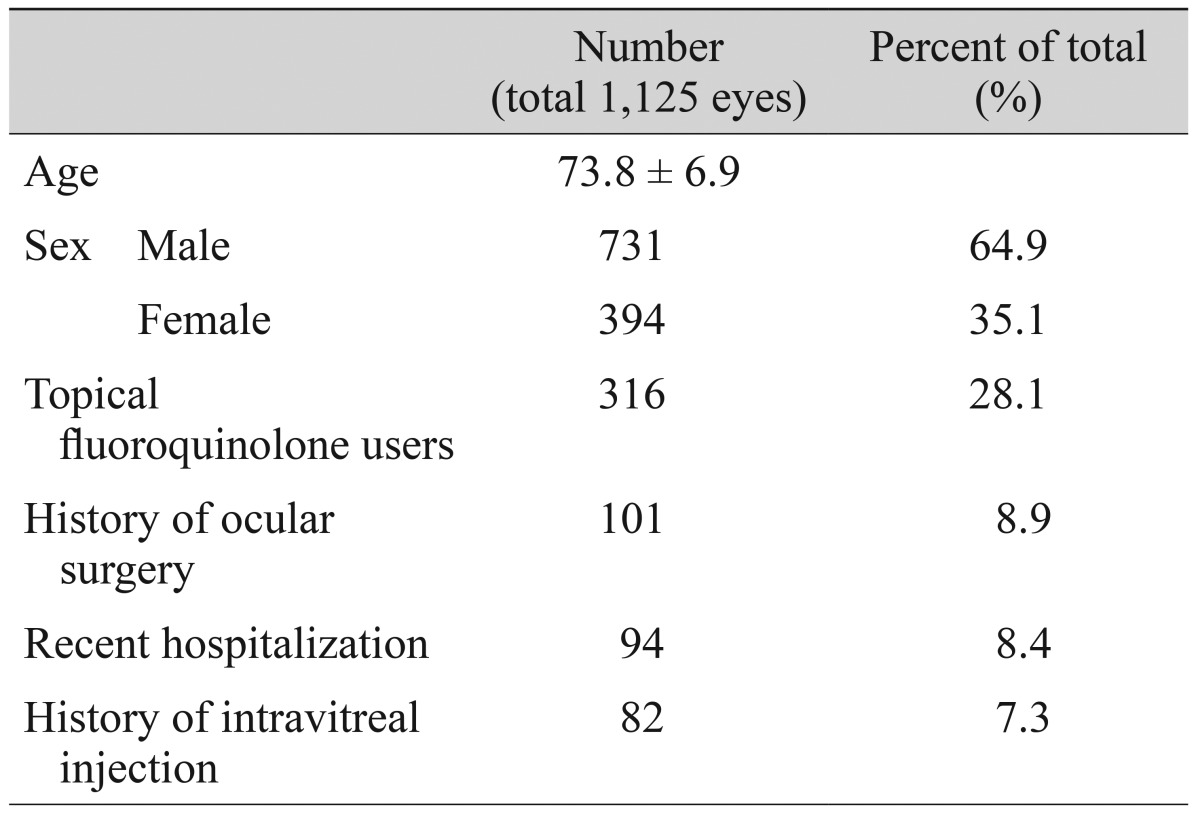
One thousand one hundred twenty-five patients were enrolled in the study. The average age of the patients was 73.8 years. A total of 731 patients (64.9%) were male, and 394 patients (35.1%) were female. Three hundred sixteen patients (28.1%) were using topical fluoroquinolone and 101 patients (8.9%) had a history of intravitreal injection. Ninety-four patients (8.4%) had a history of ocular surgery and 82 patients (7.3%) were hospitalized within 1 year. Table 1 summarizes the baseline demographics of the study population.
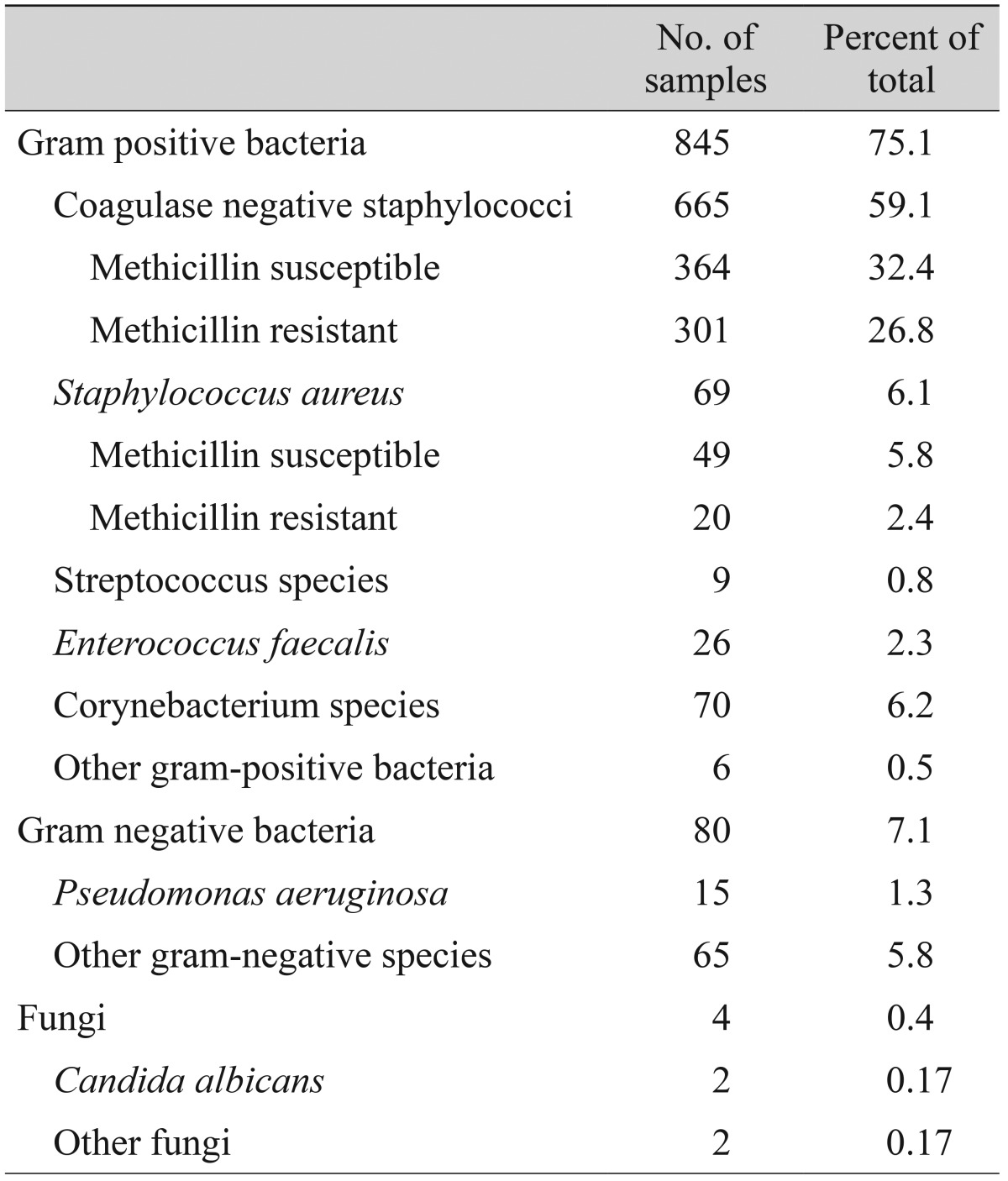
Nine hundred twenty-nine cultures were isolated from eight hundred seventy-seven eyes, and the remaining two hundred forty eight-eyes (22%) showed no growth. Table 2 summarizes the isolated organisms, which included 845 gram-positive organisms (75.1%), 80 gram-negative organisms (7.1%) and four fungi (0.3%). CNS was the most common group of bacterial organisms isolated (59.1%). Among 665 CNS isolates, 364 were methicillin susceptible (54.7%) and 301 were methicillin resistant (45.3%).
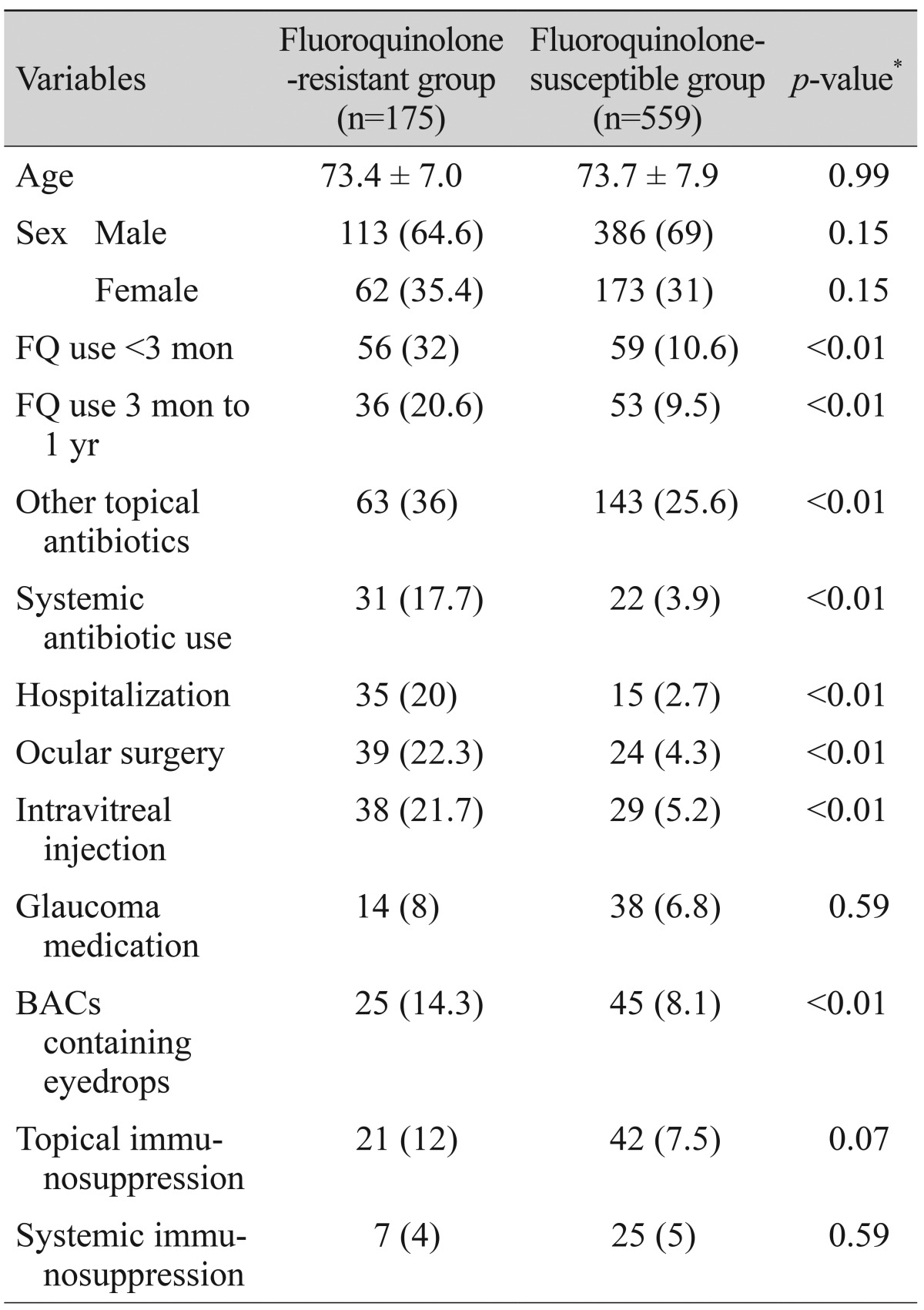
Of 734 CNS and S. aureus cultures, 175 (23.8%) were resistant to ciprof loxacin, levof loxacin, gatif loxacin or moxifloxacin. Among the various risk factors investigated, topical fluoroquinolone use within 3 months prior to culture, topical fluoroquinolone use between 3 months to 1 year, other topical antibiotic use within 1 year prior to culture, recent hospitalization, systemic antibiotic use, ocular surgery, intravitreal injection and use of eyedrops containing BACs was more frequent in resistant isolates than in susceptible isolates. Table 3 summarizes characteristics between the fluoroquinolone-resistant group and the fluoroquinolone-susceptible group.
In the multivariate regression analysis, recent ocular surgery (odds ratio [OR], 8.457), recent hospitalization (OR, 6.646), topical fluoroquinolone use within 3 months prior to culture (OR, 4.918), history of intravitreal injection (OR, 2.976), systemic antibiotic use (OR, 2.665), use of eyedrops containing BACs (OR, 2.323), topical fluoroquinolone use between 3 months to 1 year (OR, 1.943) and other topical antibiotic use within 1 year prior to culture (OR, 1.673) were statistically significant risk factors for fluoroquinolone resistance (Table 4).
There is widespread consensus that perioperative topical antibiotics during cataract surgery are effective in minimizing the incidence of endophthalmitis [1]. In this study, we investigated patterns of ocular normal flora in patients undergoing cataract surgery in the Korean population, and retrospectively reviewed medical records of the patients to analyze the factors affecting fluoroquinolone resistance. Knowing the extent and spectrum of possible ocular flora and factors affecting fluoroquinolone resistance will aid in making the most reasonable decisions regarding antibiotic choice.
The most prevalent microorganism isolated was CNS and this finding is consistent with those of previously published studies [6,9,10]. Among the CNS isolates, methicillin-resistant CNS (MRCNS) was present in 45.3%. Our result is comparable to that of a previous study, where there was 45% MRCNS in San Francisco (2004-2005) [9], 37% in South Korea (2005-2006) [6], 37.9% in Japan (2007) [11] and 47.1% in USA (2007-2008) [12]. These findings suggest that MRCNS strains have disseminated in the community. The methicillin-resistant S. aureus (MRSA) rate in our study was 28.9%, in contrast to the conjunctival MRSA rate of 22% from Miami [13], 40% from Japan [11] and 63.6% from Saint Louis [14]. These wide disparities in MRSA from region to region suggest that antibiotic resistance is different in different locations and communities.
The reason we pay particular interest to methicillin resistance is because it is often regarded as a marker for multidrug resistance and a more virulent infectious course [12]. MRSA and MRCNS isolates were more likely to be resistant to other drug classes when compared with methicillin-susceptible S. aureus and methicillin-susceptible CNS isolates [7,15]. Methicillin resistance is known to be acquired by the mecA gene, encoded by a mobile genetic element designated staphylococcal cassette chromosome mec. The mecA gene encodes a low affinity penicillin-binding protein designated PBP2' or PBP2a, which can continue bacterial cell-wall biosynthesis, even if native PBPs have been inactivated by β-lactam antibiotics [16,17]. In addition, an insertion sequence present in staphylococcal cassette chromosome mec of MRSA is considered to be involved in multidrug resistance [18].
Topical fluoroquinolones are frequently used as a prophylaxis for cataract surgery, however rapidly emerging antibiotic resistance is a great concern. Several studies have demonstrated an association between systemic fluoroquinolone use and resistance in S. aureus [19]. However, few studies have demonstrated the association between the use of topical fluoroquinolone and resistance. In this report, we conducted a study targeting S. aureus and CNS, which comprise the majority of ocular gram-positive flora and analyzed various risk factors that could possibly affect fluoroquinolone resistance.
Previous use of systemic and topical antibiotics was a risk factor for fluoroquinolone resistance [20]. One possible explanation for why prior antibiotic use might affect antibiotic resistance is that fluoroquinolones destroy susceptible strains when in contact with commensal flora, allowing the development of resistant mutants, having appeared spontaneously during bacterial replication [21]. Identification of eff lux proteins in the Nor family that regulate the secretion of fluoroquinolones, such as NorA, NorB, and NorC, were also shown to be due to another mechanism for fluoroquinolone resistance in S. aureus and CNS [22].
History of hospitalization was associated with fluoroquinolone resistance. This correlation was confirmed by many previous reports [23]. Patients and residents in healthcare facilities are more prone to be infected or colonized, and cross transmission is crucial in spreading bacteria through healthcare personnel [24]. Furthermore, hospitalized patients might have been involved with administration of systemic antibiotics as well as systemic immunosuppression, thus leading to f luoroquinolone resistance [23]. A history of ocular surgery and intravitreal injection were associated with fluoroquinolone resistance and this correlation could be explained by the use of topical antibiotics prior to or after the procedure.
We report an interesting finding that has not yet been reported. Use of eyedrops containing BACs is correlated with fluoroquinolone resistance. BACs are a widely used class of quaternary ammonium compounds especially in the field of ophthalmology as disinfectants for eyedrops. Although several published reports have demonstrated that disinfectants and antiseptics resulted in increased tolerance to these antimicrobials without evidence of increased antibiotic resistance [25], there is a significant body of literature that suggests otherwise [26,27]. Exposure of bacteria to BACs potentially facilitates resistance to these compounds as well as to the clinically important antibiotics by virtue of acquisition and proliferation of antibiotic resistance genes, which are associated with a number of antibiotic resistant mechanisms, including the multidrug efflux pumps [26,27,28].
Our study had several limitations. Generalization of our results to the national level was difficult. The age of our patients (mean age, 73.8 years; standard deviation, 6.9 years) was not representative of the general population and the male-to-female ratio of the subjects was 6.5 to 3.5, due to the disproportional in-patient and out-patient male ratio (over 90%) of the Veterans Health Service Medical Center. Although study results might not represent the general population, the results do reflect the characteristics of the patients typically undergoing cataract surgery who are in their sixth to eighth decades.
We collected the data retrospectively, so our study might be exposed to measurement bias and misclassification errors. Finally, we cannot exclude the role of the environment on the emergence of resistant bacteria, including factors such as contact at home with a person at risk, international travels, foods, and other factors [29].
In conclusion, previous topical fluoroquinolone and other topical antibiotic use, systemic antibiotic use, hospitalization, ocular surgery, intravitreal injection and use of eyedrops containing BACs were identified as independent risk factors for fluoroquinolone resistance in the patients undergoing cataract surgery. The identification of these independent risk factors could be helpful in choosing perioperative empirical antibiotic when planning cataract surgery and initiating an empiric antibiotic therapy for suspected endophthalmitis. Furthermore, the risk factors identified in this study can be utilized to prevent the emergence and spread of fluoroquinolone resistance in ocular flora.
Notes
References
1. Endophthalmitis Study Group. European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007; 33:978–988. PMID: 17531690.
2. Liesegang TJ. Use of antimicrobials to prevent postoperative infection in patients with cataracts. Curr Opin Ophthalmol. 2001; 12:68–74. PMID: 11150084.

3. Ou JI, Ta CN. Endophthalmitis prophylaxis. Ophthalmol Clin North Am. 2006; 19:449–456. PMID: 17067900.
4. Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991; 98:1769–1775. PMID: 1775308.

5. Gordon-Bennett P, Karas A, Flanagan D, et al. A survey of measures used for the prevention of postoperative endophthalmitis after cataract surgery in the United Kingdom. Eye (Lond). 2008; 22:620–627. PMID: 17173008.

6. Chung JL, Seo KY, Yong DE, et al. Antibiotic susceptibility of conjunctival bacterial isolates from refractive surgery patients. Ophthalmology. 2009; 116:1067–1074. PMID: 19395038.

7. Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008; 145:951–958. PMID: 18374299.

8. Chang DF, Braga-Mele R, Mamalis N, et al. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg. 2007; 33:1801–1805. PMID: 17889779.
9. Ta CN, Chang RT, Singh K, et al. Antibiotic resistance patterns of ocular bacterial flora: a prospective study of patients undergoing anterior segment surgery. Ophthalmology. 2003; 110:1946–1951. PMID: 14522770.
10. Park SH, Lim JA, Choi JS, et al. The resistance patterns of normal ocular bacterial flora to 4 fluoroquinolone antibiotics. Cornea. 2009; 28:68–72. PMID: 19092409.

11. Hori Y, Nakazawa T, Maeda N, et al. Susceptibility comparisons of normal preoperative conjunctival bacteria to fluoroquinolones. J Cataract Refract Surg. 2009; 35:475–479. PMID: 19251140.

12. Olson R, Donnenfeld E, Bucci FA Jr, et al. Methicillin resistance of Staphylococcus species among health care and nonhealth care workers undergoing cataract surgery. Clin Ophthalmol. 2010; 4:1505–1514. PMID: 21191448.

13. Alabiad CR, Miller D, Schiffman JC, Davis JL. Antimicrobial resistance profiles of ocular and nasal flora in patients undergoing intravitreal injections. Am J Ophthalmol. 2011; 152:999–1004.e2. PMID: 21861973.

14. Hsu HY, Lind JT, Tseng L, Miller D. Ocular flora and their antibiotic resistance patterns in the midwest: a prospective study of patients undergoing cataract surgery. Am J Ophthalmol. 2013; 155:36–44.e2. PMID: 22995030.

15. Haas W, Pillar CM, Torres M, et al. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol. 2011; 152:567–574.e3. PMID: 21652021.

16. Hartman BJ, Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984; 158:513–516. PMID: 6563036.

17. Ito T, Katayama Y, Hiramatsu K. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob Agents Chemother. 1999; 43:1449–1458. PMID: 10348769.
18. Barberis-Maino L, Berger-Bachi B, Weber H, et al. IS431, a staphylococcal insertion sequence-like element related to IS26 from Proteus vulgaris. Gene. 1987; 59:107–113. PMID: 2830163.

19. Rogues AM, Dumartin C, Amadeo B, et al. Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals. Infect Control Hosp Epidemiol. 2007; 28:1389–1395. PMID: 17994520.
20. Fintelmann RE, Hoskins EN, Lietman TM, et al. Topical fluoroquinolone use as a risk factor for in vitro fluoroquinolone resistance in ocular cultures. Arch Ophthalmol. 2011; 129:399–402. PMID: 21482865.

21. Chenia HY, Pillay B, Pillay D. Analysis of the mechanisms of fluoroquinolone resistance in urinary tract pathogens. J Antimicrob Chemother. 2006; 58:1274–1278. PMID: 17040923.

22. Juarez-Verdayes MA, Parra-Ortega B, Hernandez-Rodriguez C, et al. Identification and expression of nor efflux family genes in Staphylococcus epidermidis that act against gatifloxacin. Microb Pathog. 2012; 52:318–325. PMID: 22426170.
23. Lautenbach E, Fishman NO, Bilker WB, et al. Risk factors for fluoroquinolone resistance in nosocomial Escherichia coli and Klebsiella pneumoniae infections. Arch Intern Med. 2002; 162:2469–2477. PMID: 12437407.

24. Richard P, Delangle MH, Merrien D, et al. Fluoroquinolone use and fluoroquinolone resistance: is there an association. Clin Infect Dis. 1994; 19:54–59. PMID: 7948558.

25. Weber DJ, Rutala WA. Use of germicides in the home and the healthcare setting: is there a relationship between germicide use and antibiotic resistance? Infect Control Hosp Epidemiol. 2006; 27:1107–1119. PMID: 17006819.

26. Braoudaki M, Hilton AC. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J Clin Microbiol. 2004; 42:73–78. PMID: 14715734.
27. Mc Cay PH, Ocampo-Sosa AA, Fleming GT. Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture. Microbiology. 2010; 156:30–38. PMID: 19815578.

28. Buffet-Bataillon S, Tattevin P, Bonnaure-Mallet M, Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds: a critical review. Int J Antimicrob Agents. 2012; 39:381–389. PMID: 22421329.
29. Vincent C, Boerlin P, Daignault D, et al. Food reservoir for Escherichia coli causing urinary tract infections. Emerg Infect Dis. 2010; 16:88–95. PMID: 20031048.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download