Abstract
Purpose
To assess whether the expression of heat shock protein 72 (Hsp72) protects rat retinal ganglion cells (RGC-5) from apoptotic cell death.
Methods
Hsp72 expression in RGC-5 cells transduced with replication-deficient recombinant adenovirus was analyzed by Western blot analysis and immunofluorescence. The effect of Hsp72 expression on etoposide-induced apoptotic cell death was examined by microscopic analysis and confirmed by cell proliferation assay.
Results
Western blot analysis and immunofluorescence clearly showed adenovirus-mediated Hsp72 expression in RGC-5 cells. Treatment with etoposide resulted in the death of a proportion of the cells by apoptosis. However, this apoptotic cell death was significantly reduced in cells expressing Hsp72, with the reduction in cell death correlating to the level of Hsp72 expression.
Glaucoma is a complex ocular disease characterized by an optic neuropathy with excavation of the head of the optic nerve [1]. The disease is the second most common cause of bilateral blindness, affecting over 67 million people worldwide. A hallmark of glaucoma is the progressive death of retinal ganglion cells (RGCs), which is also a fundamental pathologic event in a number of other ophthalmic diseases such as diabetic retinopathy. Physiologic axotomy, excitotoxicity, oxidative stress, and other undefined factors are common mechanisms involved in the death of RGCs [2,3].
Heat shock proteins (Hsps) are evolutionarily well-conserved, ubiquitous proteins found in all cells in all forms of life. Hsps are normally present at low levels but their expression is rapidly increased in response to a variety of harmful stimuli including hypoxia, ischemia, or other types of metabolic stress as well as temperature elevation. This phenomenon, known as the stress response, is thought to be vital in the intracellular chaperoning function to assist the proper folding of misfolded or mutated proteins and thereby protect cells from death [4,5].
The present study investigated whether recombinant adenovirus-mediated expression of 72 kDa heat shock protein (Hsp72) can protect cultured RGCs from apoptotic cell death. Hsp72, the inducible member of the 70 kDa family, is one of the most abundant and best characterized subsets of eukaryotic stress proteins [6]. Hsp72 is known to be essential to cytoprotection after the stressful stimuli described above and therefore served as an attractive candidate for gene therapy of degenerative disease including glaucoma [7-9].
A replication-deficient recombinant adenovirus expressing human Hsp72 was constructed using the AdEasy system (Qbiogene, Carlsbad, CA, USA). The cDNA for Hsp72 (approved gene symbol HSPA1A) was amplified by reverse transcription-polymerase chain reaction (PCR) using total RNA extracted from HeLa cells that were previously heat shocked at 42℃ for 30 minutes. The amplified product was cloned into the pShuttle-CMV vector and verified by sequencing. The vector was then transfomed into Escherichia coli strain BJ5183 together with the pAdEasy-1 vector that contains the entire adenovirus serotype 5 genome except the E1 and E3 regions. The recombinant adenoviral constructs were selected and transfected into the HEK-293 packaging cell line to produce viral particles. Viral titers were determined by limiting dilution on 293 cells and the absence of the E1a gene in the viral constructs was confirmed by PCR.
The rat retinal ganglion cell line, RGC-5, was originally provided as a gift from Dr. Neeraj Agarwal (University of North Texas Health Science Center, Fort Worth, TX, USA). RGC-5 cells were maintained in medium containing Dulbecco's modified Eagle's medium (Life Technologies, Rockville, MD, USA), 10% fetal bovine serum, and antibiotics at 37℃ in a humidified 5% CO2-95% air atmosphere.
RGC-5 cells grown to 70% confluence in a 24-well plate were transduced with a recombinant adenovirus expressing Hsp72 for 2 hours at a multiplicity of infection (MOI) of 5 to 50 plaque forming units (pfu) per cell. Forty-eight hours after transduction, adherent cells were lysed with 0.4 mL of 1 × Laemmli sample buffer and boiled for 5 minutes. The protein samples were resolved by sodium dodesyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was blocked with 5% skim milk overnight, incubated for 2 hours with anti-Hsp72 antibody (Stressgen, Victoria, Canada), and reacted for 2 hours with peroxidase-conjugated anti-mouse antibody (Amersham, Buckinghamshire, UK), followed by being probed with a chemiluminescence kit (Amersham).
RGC-5 cells grown in a chamber slide were transduced for 2 hours at an MOI of 20 pfu per cell. Forty-eight hours post-transduction, the cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and blocked with 2% bovine serum albumin. The cells were then incubated for 2 hours with anti-Hsp72 antibody, reacted for 2 hours with Alexa Fluor 568-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, OR, USA), and counterstained with 4',6-diamidino-2-phenylindole.
RGC-5 cells were dispensed in a 96 well plate at a concentration of 1 × 104 cells per well and transduced for 2 hours at an MOI of 5 and 20 pfu. The cells were washed, replenished with 100 µL of media containing 5 to 20 µM etoposide and cultured further. After 48 hours, 10 uL of reagent for used in the cytotoxicity assay (WST-8; Dojindo, Rockville, MD, USA) per well was added, and the absorbance at 450 nm was read by an automatic microplate reader after 1 hour of incubation.
To express Hsp72 exogenously in RGC-5 cells that are not easily transfected by conventional methods, the cells were transduced with a recombinant adenovirus expressing human Hsp72. Western blot analysis showed that protein bands of 72 kDa corresponding to Hsp72 were clearly detected in lysates of cells transduced with a recombinant adenovirus expressing human Hsp72. The intensities of the bands were found to be in proportion to the degree of viral transduction. In untransduced control cells, a faint band equivalent to endogenous Hsp72 expression was barely detectable (Fig. 1A). Immunocytochemistry yielded consistent results showing that cytoplasmic staining for characteristic Hsp72 expression could be detected exclusively in transduced cells but not in untransduced cells, confirming the adenovirus-mediated Hsp72 gene expression (Fig. 1B).
To examine the effect of Hsp72 on the death of RGC-5 cells caused by apoptosis, the cells were treated with etoposide immediately after viral transduction. Compared with control cells (Fig. 2A), early cell death was seen in a small proportion of cells in the culture without serum (Fig. 2B), and the degree of cell death was accelerated by the treatment of etoposide (Fig. 2C). However, the etoposide-induced cell death appeared to be blocked by Hsp72 expression, as observed by the enhanced viability of cells that were transduced immediately before etoposide treatment (Fig. 2D). To confirm the effect of Hsp72 on the survival of RGC-5 cells, a cell proliferation assay was performed on cells treated with etoposide following viral transduction (Fig. 3). The rate of viable cells was reduced by 44.8 ± 6.7% and 80.8 ± 9.2% relative to control cells at 5 and 20 µM etoposide, respectively. Viral transduction itself might be toxic to cells because cell viability was slightly decreased when Hsp72 was expressed in control cells. The Hsp72 expression, however, seemed to be protective to cells undergoing apoptosis in that the rate of cell death in the cultures exposed to etoposide was progressively reduced as the degree of viral transduction increased. At the highest level of Hsp72 expression, the number of viable cells recovered was increased by 24.0 ± 3.1% and 94.1 ± 10.3% over that in the control cultures at 5 and 20 µM etoposide, respectively.
In this study, we addressed whether Hsp72 can protect RGC-5 cells from apoptotic cell death. RGC-5 cells are the only immortalized RGC cell line established to date and are used widely in glaucoma research, although it is uncertain whether the cell line is of mouse or rat origin [10]. To investigate directly the effect of Hsp72 on the death of RGC-5 cells, it is necessary to express the protein exogenously in the cells. Like other cell lines, however, RGC-5 cells show very low transfection efficiency. To overcome this obstacle, we constructed a recombinant adenovirus in which a human Hsp72 cDNA is under the control of the human cytomegalovirus promoter for its constitutive expression and demonstrated that the viral vector was successful in both transducing RGC-5 cells and expressing its transgene inside the cells. We showed that the degree of cell death by etoposide was inversely proportional to the degree of viral transduction in the cells. Etoposide, an inhibitor of topoisomerase II, is a well-known apoptosis-inducing reagent [11]. Therefore, our results present direct evidence for the cytoprotective ability of Hsp72 against apoptotic cell death.
The protective role of heat shock was first described in light-damaged rat retina and Hsp72 protein was identified as the entity primarily responsible for the response [12,13]. A growing body of evidence has accumulated that induction of Hsp72 is essential for neuronal tolerance to a wide variety of stressful conditions including ischemic or excitotoxic insults, seizure, oxidative stress, and apoptotic stimuli [5,8,9]. The present study demonstrates that Hsp72 expression itself is sufficient to protect RGC-5 cells from apoptotic cell death. Apoptosis, or programmed cell death, of RGCs is one of the earlier signs of the pathogenesis of glaucoma. Because inhibition of RGC death or a halt of its progression is the key to glaucoma treatments, the ability of Hsp72 to intervene in the process of apoptosis is attractive as a novel therapeutic intervention in glaucoma. Gene therapy, which is a practical alternative to current therapies that are limited to reduction of elevated intraocular pressure, is an approach by which Hsp72 could be useful in a clinical context. Recent studies reported that Hsp72 induction by zinc or geranylgeranylacetone administration increased RGC survival in a rat glaucoma model, suggesting that Hsp72 could be therapeutic for glaucoma [14,15].
The eye may perhaps be the first organ for which gene therapy is used routinely in a clinical setting because it has advantages such as well-defined anatomy with small size, translucent media allowing excellent visual localization, and easy accessibility by which therapeutic agents can be delivered to the vicinity of cells with a particular defect [16]. The replication-deficient adenovirus generated in this study is a sophisticated gene delivery tool and such viral-based vectors are expected to have enormous potential as a mammalian expression system for gene therapy.
Recently, it has been reported that Hsp72 can be identified in the extracellular compartments and the extracellular Hsp72 acts as a cytokine that induces inflammation and modulates the innate immune response [17-20]. The dual role of Hsp72 as a molecular chaperone and cytokine suggests that fine tuning its expression may be a potential route for a novel therapeutic approach to glaucoma and other neurodegenerative diseases.
Figures and Tables
Fig. 1
Recombinant adenovirus-mediated Hsp72 gene transfer into RGC-5 cells. (A) A representative Western blot is shown of Hsp72 expression in cells transduced with an adenoviral vector carrying human Hsp72 cDNA at the indicated multiplicity of infection (MOI). (B) Representative photomicrographs are shown of Hsp72 expression (red) in untransduced cells (right) or cells transduced with an adenoviral vector at an MOI of 20 plaque forming units (pfu) per cell (left). Blue fluorescence represents nuclei stained with 4',6-diamidino-2-phenylindole. Bar = 50 µm.

Fig. 2
Effect of Hsp72 expression on etoposide-induced cell death. Typical photomicrographs of RGC-5 cells were taken with a phase-contrast microscope after 48 hours of culture with (A) media containing 10% serum, (B) media without serum, (C) media without serum but containing 20 µM etoposide, or (D) media without serum but containing an adenoviral stock equivalent to an multiplicity of infection of 20 plaque forming units per cell as well as 20 µM etoposide. Bar = 100 µm.
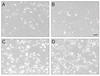
Fig. 3
Quantitation of Hsp72-induced protection against etoposide-induced cell death. Cells were transduced with an adenovirus and cultured with serum-free media containing etoposide. After 48 hours, the cell proliferation rate was analyzed colorimetrically using a cell counting kit employing WST-8. Similar results were obtained in three separate experiments. Each column and bar represents the mean ± SEM from four wells. *p < 0.01, **p < 0.001 vs. control (Student's t-test). MOI = multiplicity of infection.

Acknowledgements
This work was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund, KRF-2008-521-E00078), by a grant from the Korea Science and Engineering Foundation Science Research Center project endowed to the Molecular Therapeutic Research Center, and by a Samsung Biomedical Research Institute grant (#SBRI C-A8-206-1).
References
1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996. 80:389–393.
2. Shieds MB, Ritch R, Krupin T. Ritch R, Shields MB, Krupin T, editors. Classification of the glaucoma. The glaucomas. 1996. 2nd ed. St. Louis: Mosby;717–725.
3. Shieds MB. Textbook of glaucoma. 1998. 4th ed. Baltimore: Williams & Wilkins;153–176.
4. Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998. 80:183–201.
5. Beere HM. "The stress of dying": the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004. 117(Pt 13):2641–2651.
6. Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996. 1:23–28.
7. Amin V, Cumming DV, Latchman DS. Over-expression of heat shock protein 70 protects neuronal cells against both thermal and ischaemic stress but with different efficiencies. Neurosci Lett. 1996. 206:45–48.
8. Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70). Mol Med Today. 1999. 5:525–531.
9. Tezel G, Yang J, Wax MB. Heat shock proteins, immunity and glaucoma. Brain Res Bull. 2004. 62:473–480.
10. Van Bergen NJ, Wood JP, Chidlow G, et al. Recharacterization of the RGC-5 retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2009. 50:4267–4272.
11. Mizumoto K, Rothman RJ, Farber JL. Programmed cell death (apoptosis) of mouse fibroblasts is induced by the topoisomerase II inhibitor etoposide. Mol Pharmacol. 1994. 46:890–895.
12. Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988. 241:1817–1820.
13. Rordorf G, Koroshetz WJ, Bonventre JV. Heat shock protects cultured neurons from glutamate toxicity. Neuron. 1991. 7:1043–1051.
14. Park KH, Cozier F, Ong OC, Caprioli J. Induction of heat shock protein 72 protects retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2001. 42:1522–1530.
15. Ishii Y, Kwong JM, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003. 44:1982–1992.
16. Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002. 28:267–275.
17. Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000. 6:435–442.
18. Guzhova I, Kislyakova K, Moskaliova O, et al. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001. 914:66–73.
19. Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002. 2:185–194.
20. Calderwood SK, Mambula SS, Gray PJ Jr. Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci. 2007. 1113:28–39.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download