Abstract
A 60-year-old woman who had experienced two episodes of amaurosis fugax in her right eye presented with vision loss. Two weeks earlier, at a private clinic, she was diagnosed with impending central retinal vein occlusion (CRVO) of the right eye and received an intravitreal injection of bevacizumab. Two weeks after this injection she was diagnosed with ischemic CRVO. At 11-weeks post-presentation, extremely ischemic features were observed with fluorescein angiographic findings of severe vascular attenuation and extensive retinal capillary obliteration. At 22-weeks post-presentation she was diagnosed with neovascular glaucoma; she experienced no visual improvement over the following several months.
We report a case of ischemic central retinal vein occlusion (CRVO) in a patient who initially had impending CRVO and who was subsequently treated with intravitreal bevacizumab.
A 60-year-old woman with a history of normal tension glaucoma presented because of vision loss in her right eye. Four weeks prior to her first visit to our hospital she had noted a sudden blurring over the entire visual field of her right eye. This lasted for approximately ten minutes and then fully recovered; eight days later, she experienced exactly the same phenomenon. Her previous clinical history was only significant for chronic hepatitis B. She did not have hypertension, diabetes mellitus, or hyperlipidemia.
She visited a private clinic on November 1, 2006. On examination, mild dilatation and tortuosity of the retinal veins, some intraretinal hemorrhages at the posterior pole, and a slightly swollen optic disc with small hemorrhages at the disc margin were found in her right eye (Fig. 1A). Her best corrected visual acuity (BCVA) was 20/20, and her intraocular pressure (IOP) was 12 mmHg. Fluorescein angiography (FAG) revealed normal choroidal filling and slightly delayed filling of the central retinal vein without capillary nonperfusion. The left eye was normal. She was diagnosed with impending CRVO and underwent a trial of intravitreal bevacizumab (2.5 mg in 0.1 mL) in an attempt to improve the vascular stasis.
She was referred to our hospital two weeks after the bevacizumab injection, on November 13. Her BCVA was 20/80, and the IOP was normal in her right eye. Fundus examination revealed numerous retinal hemorrhages in four quadrants, dilated and tortuous retinal veins, and severe disc swelling. Newly developed macular edema and cotton-wool spots were observed (Fig. 1B). FAG revealed normal choroidal circulation, a marked delay in arteriovenous transit time, and extensive areas of capillary nonperfusion. Optical coherence tomography revealed increased retinal thickness in the macular area. Electroretinography revealed decreased b-wave amplitude, but a normal a-wave. We diagnosed ischemic CRVO and continued to observe the patient without additional treatment. Laboratory data were unremarkable, including hemoglobin, platelet count, white cell count, electrolytes, and lipid profile. Additional examinations were subsequently performed; inflammatory markers and plasma viscosity were unremarkable.
At three-weeks post-presentation, on November 20, the patient noted a significant decrease in visual acuity; her BCVA had declined to counting fingers. A slit lamp examination showed a relative afferent pupillary defect. At six-weeks post-presentation, on December 15, her BCVA was unchanged and her IOP was 14 mmHg. A fundus examination revealed reductions in retinal hemorrhage and cotton-wool spots, moderate macular edema, and a somewhat sclerotic retinal vasculature. She received a prescription for aspirin 325 mg/day (acetylsalicylic acid). FAG was not performed on this occasion.
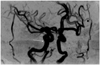
The patient was observed for another five weeks. At 11-weeks post-presentation, on January 22, her BCVA was counting fingers and her IOP was 14 mmHg. A slit lamp examination showed a rubeosis iridis. An ophthalmoscopic examination revealed opacification of the posterior pole, severe attenuation of the retinal vessels, and optic disc pallor. The macular edema had nearly resolved (Fig. 1C). Serial FAG revealed severe attenuation of the retinal vessels, extensive retinal capillary obliteration, and late staining of the optic disc, suggesting an aggravation to the extremely ischemic form (Fig. 1D). Magnetic resonance angiography showed no significant stenosis in the ipsilateral carotid artery (Fig. 2). Her right eye was immediately treated with panretinal photocoagulation.
On March 5, at 22-weeks post-presentation, the patient developed painful neovascular glaucoma, refractory to topically administered IOP reducing agents. Her visual acuity had declined to hand movements, and her IOP was 65 mmHg. Considerable iris neovascularization was evident, and a slit-lamp examination revealed signs of inflammation, including mutton fat keratic precipitates, aqueous flare, and posterior synechiae. The patient was treated with prednisolone acetate four times a day, 1% atropine twice a day, and topical antiglaucoma agents. Despite maximal antiglaucoma medication, her right eye showed no visual recovery and her IOP remained uncontrolled.
We report a case of ischemic CRVO that initially presented as impending CRVO. Impending CRVO is an uncommon condition, which may resolve or progress to complete obstruction [1]. Our patient experienced recurrent amaurosis fugax in the setting of slightly dilated and tortuous retinal veins and scattered flame-shaped hemorrhages as signs of future CRVO that became ophthalmoscopically evident within four weeks of the onset of symptoms.
The patient was given one intravitreal injection of bevacizumab (2.5 mg) prior to referral. Three weeks after this injection, her BCVA abruptly decreased to counting fingers. It is difficult to assess the exact effect of intravitreal bevacizumab on her condition. Regarding the drug itself, vascular endothelial growth factor (VEGF) is known to have a neurotrophic effect, and blockage of all VEGF isoforms by bevacizumab may create toxic effects [2]. Neuronal tissue, including the neurosensory retina, may be vulnerable to complete VEGF inhibition, especially in the presence of a disease like CRVO. A recent case report of ischemic CRVO which progressed from nonischemic CRVO following intravitreal bevacizumab injection supports this hypothesis [3].
However, the effects of bevacizumab must be interpreted cautiously in this case. Although the natural course of impending CRVO is not well established, a natural progression to ischemic CRVO is highly probable. Moreover, our patient had normal tension glaucoma that may have facilitated the CRVO. Further investigation regarding possible negative effects of intravitreal bevacizumab in ischemic conditions is needed.
Figures and Tables
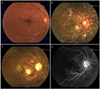
Fig. 1
The right eye at presentation and at two- and 11-weeks post-presentation. (A) Dilated and tortuous retinal veins, some intraretinal hemorrhages at the posterior pole, and a slightly swollen optic disc were found in the right eye at presentation. (B) At two-weeks post-presentation, an ophthalmoscopic examination revealed an increase in intraretinal hemorrhages, more severe disc swelling, macular edema, and newly developed cotton-wool spots. (C) At 11-weeks post-presentation, an ophthalmoscopic examination revealed whitening of the posterior pole, vascular compromise with severe attenuation, and optic disc pallor. (D) Midphase fluorescein angiography at 11-weeks post-presentation revealed extensive retinal capillary obliteration and severe attenuation of the retinal vessels.
References
1. Gass JD. Stereoscopic atlas of macular diseases: diagnosis and treatment. 1997. 4th ed. St Louis: Mosby;546–555.
2. Ziemssen F, Luke M, Messias A, et al. Safety monitoring in bevacizumab (Avastin) treatment: retinal function assessed by psychophysical (visual fields, colour vision) and electrophysiological (ERG/EOG) tests in two subgroups of patients. Int Ophthalmol. 2008. 28:101–109.
3. Kim KS, Chang HR, Song S. Ischaemic change after intravitreal bevacizumab (Avastin) injection for macular oedema secondary to non-ischemic central retinal vein occlusion. Acta Ophthalmol. 2008. 86:925–927.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download