Abstract
Purpose
To evaluate the short-term efficacy and safety of intravitreal bevacizumab injection (IVBI) in patients with retinal angiomatous proliferation (RAP).
Methods
Seven eyes of 5 patients with RAP were included in this study. All of the eyes evidenced stage 2 RAP lesions, except for one eye with a stage 3 lesion. IVBI (1.25 mg/0.05 cc) were conducted at 4 or 6-week intervals. Complete ocular examinations, angiographic results and optical coherence tomographic findings before and after the IVBI were analyzed at baseline and upon the follow-up visits.
Results
Seven eyes were studied in 5 patients who had undergone IVBI. Partial (3 eyes) or complete (4 eyes) regression of RAP was noted after IVBI in all of the studied eyes. Visual acuity improved in 5 of the eyes, and was stable in 2 of the eyes. One eye evidenced severe intraocular inflammation after IVBI and a subsequent development of new RAP, which was controlled with vitrectomy and repeat IVBI.
Retinal angiomatous proliferation (RAP) is a distinct form of neovascular age-related macular degeneration, originating from the retinal vasculature.1-4 Although laser photocoagulation, the surgical ablation of feeding retinal arterioles and draining venules, photodynamic therapy, and transpupillary thermotherapy have previously been reported as therapeutic modalities for RAP,5-10 no effective treatment for RAP has yet been developed. It was reported in an experimental mouse study that excessive vascular endothelial growth factor expression may trigger the growth of new vessels toward the subretinal space from the deep retinal capillary plexus, and the new vessels were shown to enlarge and form a complex with other vessels.11-13 This occurs in a fashion similar to the evolution of RAP, and suggests the possibility that anti-vascular endothelial growth factor may be utilized as a treatment for RAP. However, to the best of our knowledge, no previous reports regarding this issue have yet been filed in Korea. We hereby describe the short-term effectiveness and safety profile of intravitreal bevacizumab, a full-length recombinant monoclonal antibody for vascular endothelial growth factor A, delivered via injection (IVBI)into patients with RAP.
Seven consecutive eyes of five patients who had undergone IVBI for the treatment of RAP were included in this series. The study group was comprised of one man and four women. The mean age in the study group was 71.2 years. The diagnosis of RAP was predicated on the results of fluorescein angiography and indocyanine green angiography. Six eyes were verified to have stage 2 RAP lesions, in accordance with the classifications established by Yannuzzi et al.1 The remaining eye was confirmed to have a stage 3 RAP lesion. Four eyes with RAP were subjected to IVBI as an initial treatment. Three eyes with RAP which had recurred after surgical ablation were also included in this study. Both eyes were involved in all five patients, but IVBI was not considered to be an appropriate treatment modality in the fellow eyes of 3 subjects with RPE tears after photodynamic therapy or disciform scarring. Measurements of best-corrected visual acuity, slit lamp examinations, fluorescein angiography, indocyanine green angiography, and optical coherence tomography were conducted in all patients. The median pretreatment visual acuity was 20/100 (Table 1).
Bevacizumab (Avastin®, Genentech, Inc.) 1.25 mg in 0.05 ml was injected into the superior pars plana area, 3.5 mm apart from the corneal limbus, using a 30-gauge needle, and the location of injection was then changed in the following sequence: 10 o'clock, 12 o'clock, and 2 o'clock, in order to avoid repeated injections at the same location. IVBI was initially conducted at 4 weeks and then afterward at 6-week intervals. Slit lamp examination, funduscopy, and optical coherence tomography were conducted on the same day of, but prior to, the consequent IVBI. Fluorescein angiography and indocyanine green angiography were conducted at the discretion of the treating physician, and these tests were employed in order to determine the quantity of leakage and regression of the RAP lesion. Bevacizumab was injected up to 8 times. The mean follow-up duration after the initial IVBI was 8.4 months (range 6~12 months).
On angiographic examinations conducted 5.6 months(range 4~7 months) after IVBI, partial (3 eyes) or complete (4 eyes) regressions of RAP and significant reductions of the hyperfluorescent lesions were noted in all of the study eyes. On the optical coherence tomographic examinations, a reduction in the central macular thickness, as well as a complete resolution of subretinal fluid and/or retinal pigment epithelial detachment were noted in all of the eyes on the final visit. Five eyes evidenced better visual acuity and 2 eyes evidenced stable visual acuity. Median BCVA at the last visit had improved to 20/50. The visual results of the individual eyes are provided in Table 1.
In one case (patient #5 in Table 1) the patient was subjected to vitrectomy as the consequence of severe intraocular inflammation detected two days after the fourth IVBI. The bacterial culture of the vitreous specimen revealed no growth. The intraocular inflammation subsided as the result of intravitreal antibiotics and topical and systemic steroid treatments. Two weeks after the vitrectomy, the angiogram indicated a new RAP lesion. However, the RAP lesion regressed with repeated IVBI. Otherwise, no injection-related complications were detected.
In all cases, stable or improved vision, reductions of central thickness on optical coherence tomography, and partial or complete obliterations of RAP on angiography were noted (Fig. 1). Our results indirectly bolster the notion that vascular endothelial growth factor contributes to the development of RAP. As the majority of our cases evidenced stage 2 RAP with pigment epithelial detachment, IVBI appears to work, at least in lesions of up to stage 2. As was noted in three eyes in this study, the recurrence or progression of RAP lesions after photodynamic therapy or surgical ablation are not infrequent occurrences. However, the development of such occurrences appears to be reduced as a consequence of IVBI.
Although the number of cases in this study was rather small and the observation time was limited, IVBI appears initially to constitute an effective treatment, which can improve the prognosis of RAP. IVBI should be considered as a therapeutic option, particularly in cases in which RAP is diagnosed in an early stage.
Figures and Tables
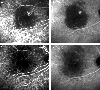
Fig. 1
Early and late phase indocyanine green angiogram (ICGA) of the right eye of the patient #2 shows focal area of hyperfluorescence and discrete rim of hypofluorescence due to retinal pigment epithelial detachment before treatment (A, B). At 5 months after intravitreal bevacizumab injection (C, D) it shows partial ablation of lesion on ICGA.
References
1. Yannuzzi LA, Negrao S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001. 21:416–434.
2. Gass JD, Agarwal A, Lavina AM, Tawansy KA. Focal inner retinal hemorrhages in patients with drusen: an early sign of occult choroidal neovascularization and chorioretinal anastomosis. Retina. 2003. 23:741–751.
3. Hartnett ME, Weiter JJ, Staurenghi G, Elsner AE. Deep retinal vascular anomalous complexes in advanced age-related macular degeneration. Ophthalmology. 1996. 103:2042–2053.
4. Kuhn D, Meunier I, Soubrane G, Coscas G. Imaging of chorioretinal anastomoses in vascularized retinal pigment epithelium detachments. Arch Ophthalmol. 1995. 113:1392–1398.
5. Blodi CF, Russell SR, Pulido JS, Folk JC. Direct and feeder vessel photocoagulation of retinal angiomas with dye yellow laser. Ophthalmology. 1990. 97:791–795.
6. Borrillo JL, Sivalingam A, Martidis A, Federman JL. Surgical ablation of retinal angiomatous proliferation. Arch Ophthalmol. 2003. 121:558–561.
7. Boscia F, Furino C, Prascina F, et al. Combined surgical ablation and intravitreal triamcinolone acetonide for retinal angiomatous proliferation. Eur J Ophthalmol. 2005. 15:513–516.
8. Boscia F, Parodi MB, Furino C, et al. Photodynamic therapy with verteporfin for retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. 2006. 244:1224–1232.
9. Bottoni F, Massacesi A, Cigada M, et al. Treatment of retinal angiomatous proliferation in age-related macular degeneration: a series of 104 cases of retinal angiomatous proliferation. Arch Ophthalmol. 2005. 123:1644–1650.
10. Kuroiwa S, Arai J, Gaun S, et al. Rapidly progressive scar formation after transpupillary thermotherapy in retinal angiomatous proliferation. Retina. 2003. 23:417–420.
11. Okamoto N, Tobe T, Hackett SF, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997. 151:281–291.
12. Lopez PF, Sippy BD, Lambert HM, et al. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996. 37:855–868.
13. Wells JA, Murthy R, Chibber R, et al. Levels of vascular endothelial growth factor are elevated in the vitreous of patients with subretinal neovascularisation. Br J Ophthalmol. 1996. 80:363–366.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download