Abstract
Purpose
This study evaluated the efficacy and safety of a brinzolamide 1%-brimonidine 0.2% fixed combination (BBFC) for normal tension glaucoma (NTG) in a South Korean population.
Methods
This study included 45 patients who were newly diagnosed with NTG and treated with BBFC as the first therapy from January 2016 through December 2016. The unilateral eye of NTG eyes of all patients were enrolled. If both eyes were eligible, the eye with the more severe glaucomatous change was selected. If the glaucomatous change was similar in both eyes, the right eye was selected. The patients received the BBFC twice a day. Diurnal intraocular pressure (IOP) was measured every 2 and 1/2 hours between 09:00 am and 04:30 pm. The IOP change with respect to body position (positional IOP) was measured at baseline and at 6 months after eyedrop instillation. Throughout the study, all side effects were recorded and monitored by the investigators.
Results
Ten patients were excluded due to an allergic reaction or follow-up loss. A total of 35 patients were enrolled in this study. The mean IOP was 15.32 ± 4.00 mmHg at baseline and 13.38 ± 3.30 mmHg at 6 months after BBFC instillation (p < 0.001). The IOP fluctuation decreased from 3.33 ± 3.10 to 2.35 ± 1.40 mmHg after BBFC instillation; however, the difference was not statistically significant (p = 0.150). The mean change in positional IOP showed a statistically significant reduction from 16.94 ± 3.18 to 14.80 ± 3.27 mmHg (p = 0.025). There was no serious adverse drug reaction except in three cases of allergic reaction.
References
1. Casson RJ, Chidlow G, Wood JP, et al. Definition of glaucoma: clinical and experimental concepts. Clin Exp Ophthalmol. 2012; 40:341–9.

2. Quigley HA, Enger C, Katz J, et al. Risk factors for the abdominal of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994; 112:644–9.
3. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:714–20.
4. Collin BH, Grabsch BE. The effect of ophthalmic preservatives on the healing rate of the rabbit corneal epithelium after keratectomy. Am J Optom Physiol Opt. 1982; 59:215–22.

5. Burstein NL. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Invest Ophthalmol Vis Sci. 1980; 19:308–13.
6. Burstein NL. Corneal cytotoxicity of topically applied drugs, ve-hicles and preservatives. Surv Ophthalmol. 1980; 25:15–30.

7. Tonjum AM. Effects of benzalkonium chloride upon the corneal epithelium studied with scanning electron microscopy. Acta Ophthalmol (Copenh). 1975; 53:358–66.
8. Boger WP 3rd. Shortterm “escape” and longterm “drift.” The dis-sipation effects of the beta adrenergic blocking agents. Surv Ophthalmol. 1983; 28(Suppl):235–42.

9. Newman-Casey PA, Robin AL, Blachley T, et al. The most abdominal barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015; 122:1308–16.
11. Aung T, Laganovska G, Hernandez Paredes TJ, et al. Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension. Ophthalmology. 2014; 121:2348–55.

12. Prata TS, De Moraes CG, Kanadani FN, et al. Posture-induced abdominal pressure changes: considerations regarding body position in glaucoma patients. Surv Ophthalmol. 2010; 55:445–53.
13. Kim HJ, Yi K. Comparison of intraocular pressures according to position using icare rebound tonometer. J Korean Ophthalmol Soc. 2014; 55:1049–55.

14. Ahn JH, Kil HK, Lee MV. Positional change of intraocular abdominal and its relationship to ocular pulse amplitude. J Korean Ophthalmol Soc. 2015; 56:234–40.
15. Shin HU, Jin SW. The efficacy of brinzolamide 1%/brimonidine 0.2% fixed combination in normal tension glaucoma. J Korean Ophthalmol Soc. 2016; 57:1619–24.

16. Gandolfi SA, Lim J, Sanseau AC, et al. Randomized trial of abdominal/brimonidine versus brinzolamide plus brimonidine for open-angle glaucoma or ocular hypertension. Adv Ther. 2014; 31:1213–27.
17. Realini T, Nguyen QH, Katz G, Dubiner H. Fixed-combination brinzolamide 1%/brimonidine 0.2% vs monotherapy with abdominal or brimonidine in patients with open-angle glaucoma or abdominal hypertension: results of a pooled analysis of two phase 3 studies. Eye. 2013; 27:841.
18. Whitson JT, Realini T, Nguyen QH, et al. Six-month results from a phase III randomized trial of fixed-combination brinzolamide 1%+ brimonidine 0.2% versus brinzolamide or brimonidine abdominal in glaucoma or ocular hypertension. Clin Ophthalmol. 2013; 7:1053–60.
19. Katz G, Dubiner H, Samples J, et al. Three-month randomized trial of fixed-combination brinzolamide, 1%, and brimonidine, 0.2%. JAMA Ophthalmol. 2013; 131:724–30.

20. King AJ, Rotchford AP. Validity of the monocular trial of abdominal pressure-lowering at different time points in patients starting topical glaucoma medication. JAMA Ophthalmol. 2016; 134:742–7.
21. King AJ, Uppal S, Rotchford AP, et al. Monocular trial of abdominal pressure-lowering medication: a prospective study. Ophthalmology. 2011; 118:2190–5.
Figure 1.
Time course data for intraocular pressure (IOP) at baseline and 6 months after brinzolamide 1%/brimonidine 0.2% fixed combination (BBFC) instillation. The mean change of IOP showed significant reduction at each time.
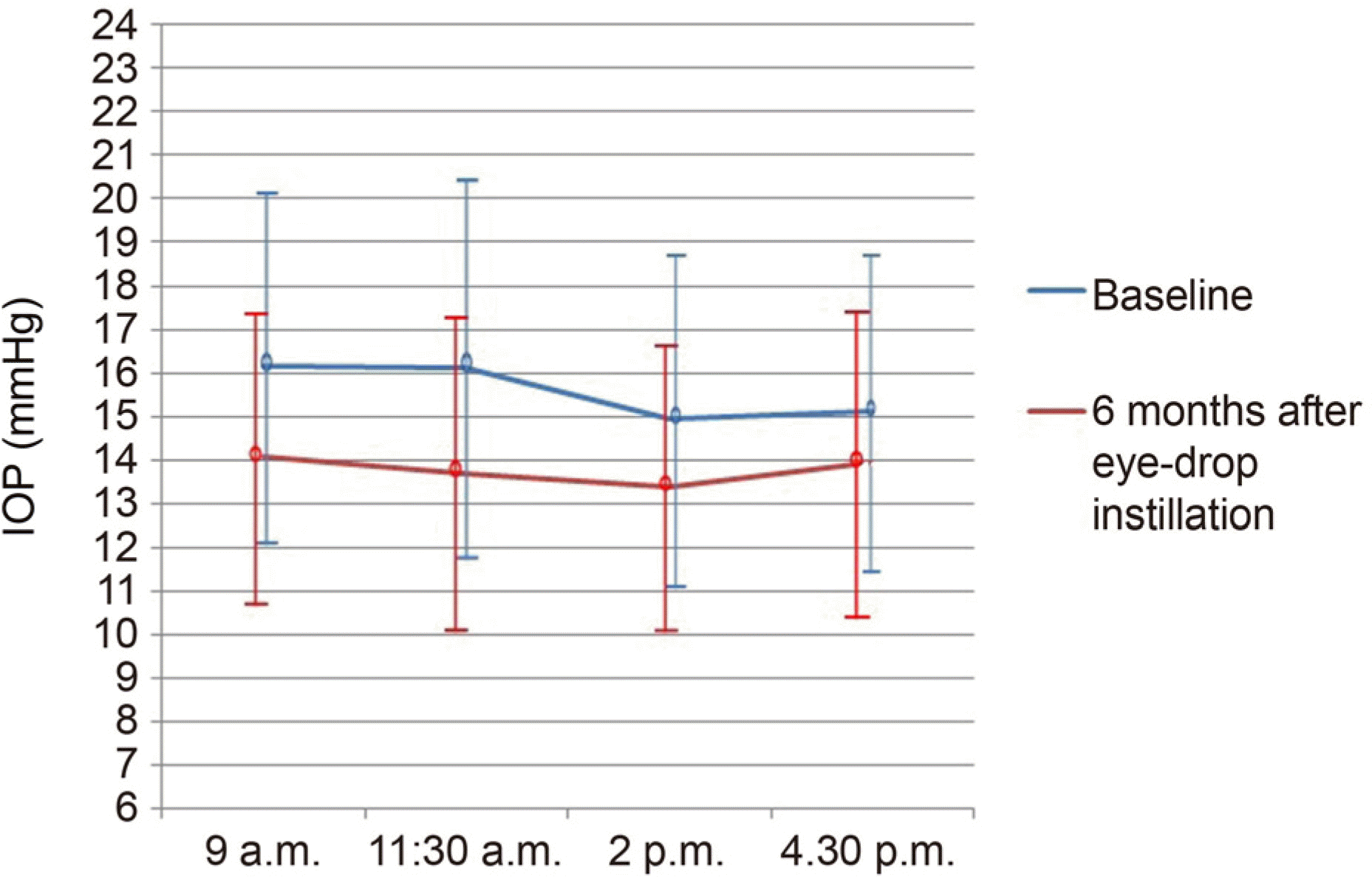
Figure 2.
Positional intraocular pressure (IOP) changes at baseline and 6 months after brinzolamide 1%/brimonidine 0.2% fixed combination (BBFC) instillation. The mean change of positional IOP showed significant reduction at each position.
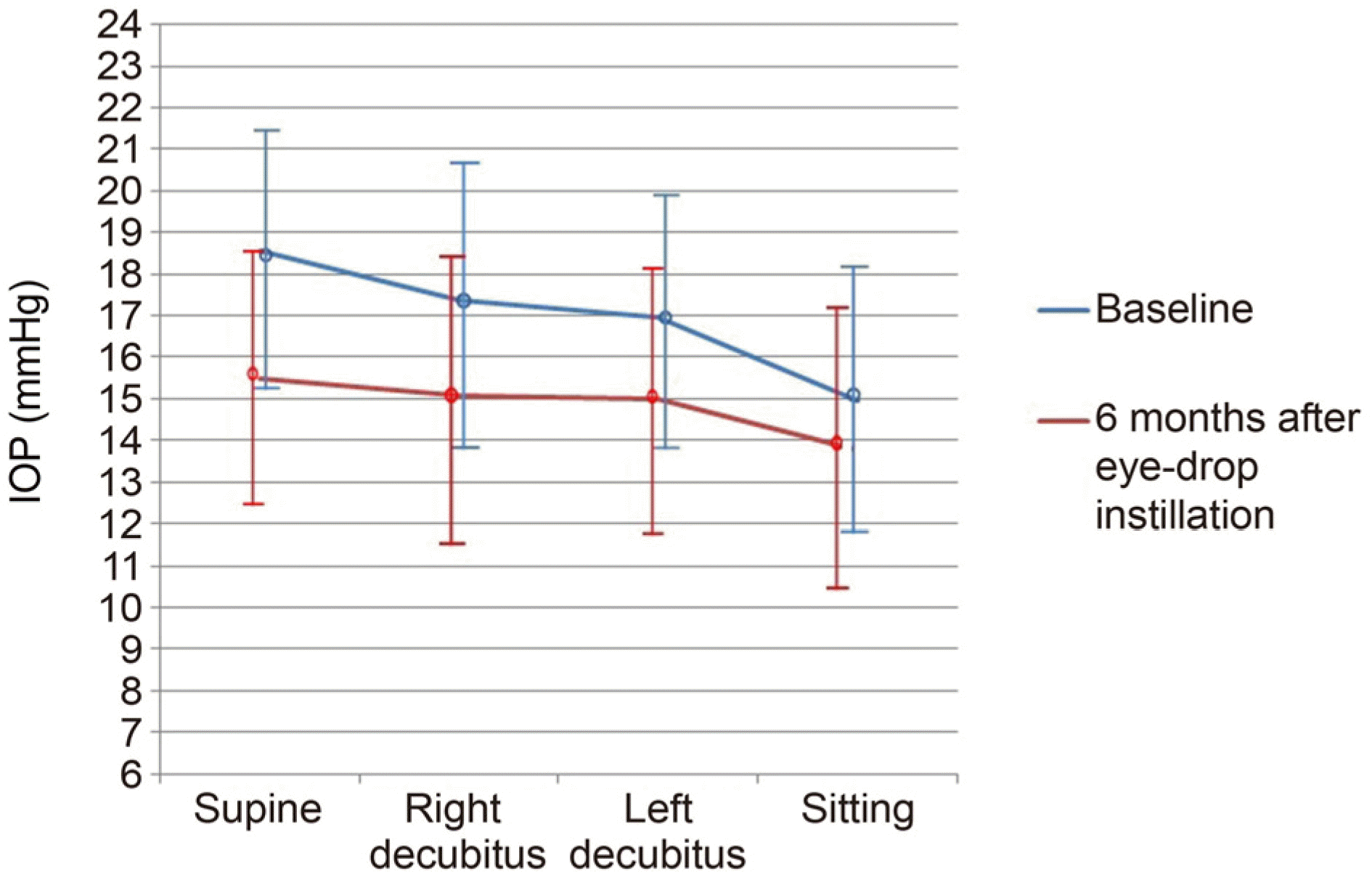
Figure 3.
Intraocular pressure (IOP) change after instillation of brinzolamide 1%/brimonidine 0.2% fixed combination (BBFC) drug. The baseline IOP was 15.32 ± 4.00 mmHg. After use of BBFC, the mean IOP was decreased to 13.62 ± 4.22 mmHg (1 month) 13.44 ± 3.80 mmHg (3 months) and 13.38 ± 3.30 mmHg (6 months).
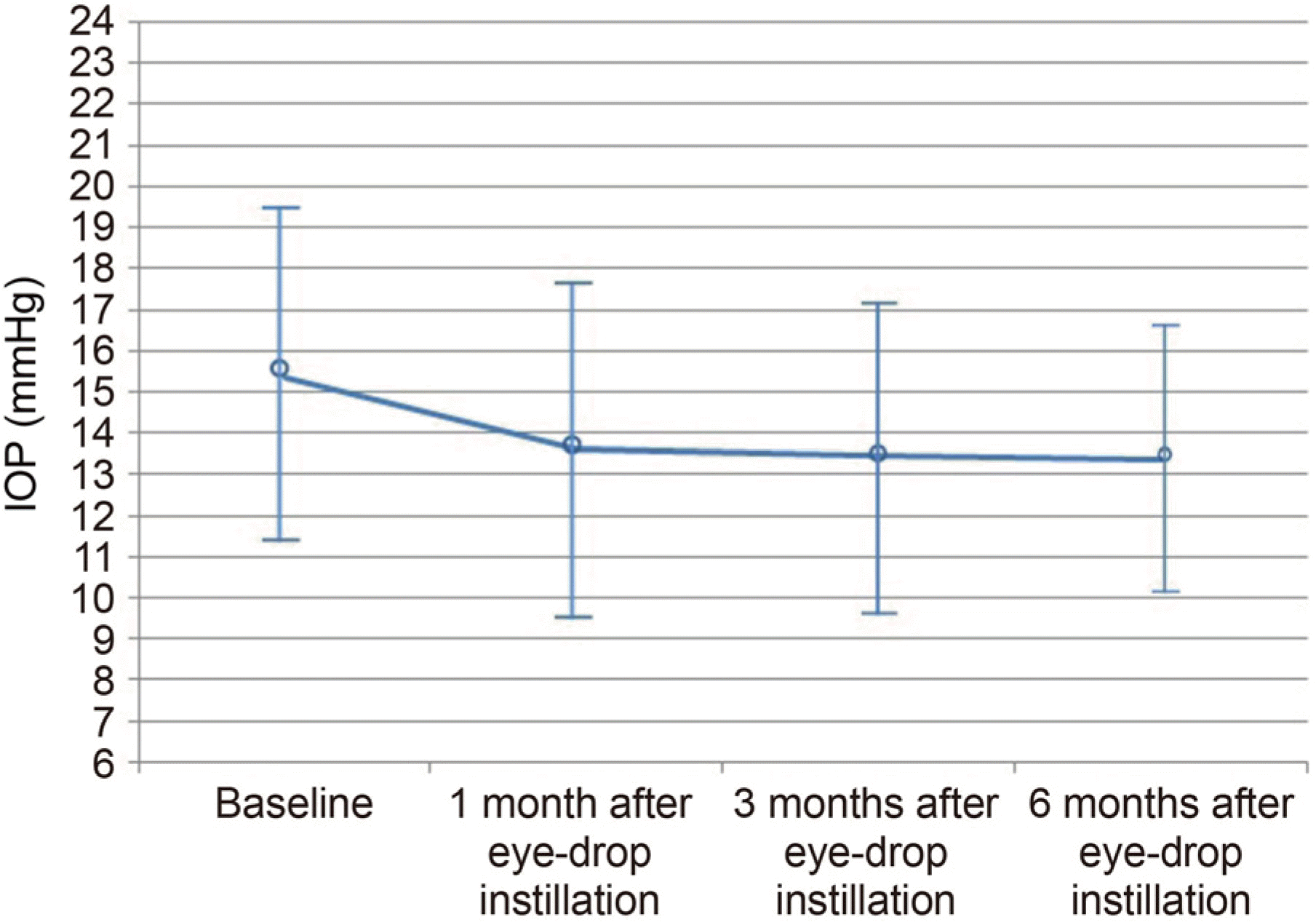
Table 1.
Profile and demograph ics of the patients
Table 2.
IOP changes at 6 months after BBFC instillation from baseline
| Diurnal IOP |
IOP (mmHg) |
Measured IOP change on (mmHg) | p-value* | |
|---|---|---|---|---|
| At baseline At 6 | months after eyedrop instillati | |||
| 9 a.m. | 16.12 ± 4.02 | 14.06 ± 3.20 | 3.09 (19.20) | <0.001 |
| 11:30 a.m. | 16.10 ± 4.47 | 13.66 ± 3.66 | 2.26 (14.08) | <0.001 |
| 2 p.m. | 14.94 ± 3.90 | 13.36 ± 3.21 | 1.60 (10.70) | 0.001 |
| 4:30 p.m. | 15.10 ± 3.52 | 13.94 ± 3.54 | 0.98 (6.48) | 0.018 |
| Mean | 15.32 ± 4.00 | 13.38 ± 3.30 | 1.94 (12.65) | <0.001 |
| IOP fluctuation (Highest IOP – Lowest IOP) | 3.33 ± 3.10 | 2.35 ± 1.40 | – | 0.150 |
Table 3.
IOP changes dependent on body position at 6 months after BBFC instillation
| Position |
IOP (mmHg) |
Measured IOP change (mmHg) (n, %) | p-value* | |
|---|---|---|---|---|
| At baseline At 6 | months after eyedrop instillation | |||
| Both eye BBFC use (n = 25 | 5)† | |||
| Supine | 18.86 ± 3.32 | 15.63 ± 3.12 | 3.15 (16.70) | <0.001 |
| Right lateral decubitus | ||||
| Right case‡ (n = 15)† | ||||
| Right eye | 17.49 ± 3.48 | 15.12 ± 3.61 | 2.37 (13.55) | 0.005 |
| Left eye | 17.19 ± 3.38 | 14.98 ± 3.50 | 2.21 (12.85) | 0.012 |
| Left lateral decubitus | ||||
| Left case‡ (n = 10)† | ||||
| Right eye | 16.69 ± 3.45 | 14.72 ± 3.05 | 1.97 (11.80) | 0.015 |
| Left eye | 16.98 ± 2.95 | 15.02 ± 2.81 | 1.96 (11.54) | 0.040 |
| Single eye BBFC use (n = | 10)† | |||
| Right BBFC eye (n = 5)† | ||||
| Supine | 18.22 ± 3.09 | 15.23 ± 2.89 | 2.99 (16.41) | 0.011 |
| Right lateral decubitus | ||||
| Right eye | 17.62 ± 3.52 | 15.45 ± 3.83 | 2.17 (12.32) | 0.042 |
| Left eye | 16.89 ± 3.08 | 14.70 ± 3.49 | 2.19 (12.97) | 0.031 |
| Left lateral decubitus | ||||
| Right eye | 17.04 ± 3.41 | 14.98 ± 3.77 | 2.06 (12.09) | 0.045 |
| Left eye | 17.32 ± 3.69 | 15.24 ± 3.73 | 2.08 (12.01) | 0.029 |
| Left BBFC eye (n = 5)† | ||||
| Supine | 18.39 ± 3.15 | 15.36 ± 3.02 | 3.03 (16.47) | 0.005 |
| Right lateral decubitus | ||||
| Right eye | 17.42 ± 3.70 | 15.49 ± 3.22 | 1.93 (11.08) | 0.035 |
| Left eye | 17.02 ± 4.01 | 15.10 ± 3.19 | 1.92 (11.28) | 0.028 |
| Left lateral decubitus | ||||
| Right eye | 16.59 ± 4.11 | 14.71 ± 3.01 | 1.88 (11.33) | 0.048 |
| Left eye | 17.22 ± 3.86 | 15.32 ± 3.17 | 1.90 (11.03) | 0.033 |
| Sitting | 14.93 ± 3.17 | 13.73 ± 3.44 | 1.23 (8.36) | 0.022 |
| Mean | 16.94 ± 3.18 | 14.80 ± 3.27 | 2.13 (12.63) | 0.025 |
Table 4.
IOP changes of dependent eye (lower position) at lateral decubitus position after BBFC instillation
| Position |
IOP (mmHg) |
Measured IOP change (mmHg) (n, %) | p-value* | |
|---|---|---|---|---|
| At baseline At 6 | months after eyedrop instillatio | |||
| Right eye BBFC use (n =20)† | ||||
| Right lateral decubitus | ||||
| Right eye | 17.52 ± 3.49 | 15.20 ± 3.67 | 2.32 (13.24) | 0.010 |
| Left eye BBFC use (n = 15)† | ||||
| Left lateral decubitus | ||||
| Left eye | 16.99 ± 3.30 | 14.83 ± 2.94 | 2.16 (12.71) | 0.026 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download