Abstract
Purpose
To evaluate the clinical effects of triamcinolone-soaked nasal packing on endonasal dacryocystorhinostomy (DCR).
Methods
The study included 91 patients (156 eyes) with primary acquired nasolacrimal duct obstruction who underwent endonasal DCR from March 2015 to February 2017. A total of 50 eyes were packed with triamcinolone-soaked Nasopore® and 106 eyes were packed with Nasopore® without triamcinolone (control group). The anatomical and functional success percentage, revision percentage, and postoperative complications such as granulation, synechiae, and membrane formation were compared between the groups at 1 week, 1 month, 2 months, and 4 months postoperatively.
Results
At postoperative 2 months, there was a statistically significant difference in the anatomical success percentage in the triamcinolone-soaked group (100%) compared to the control group (86.8%; p = 0.007). There were no statistically significant differences between the two groups in anatomical success percentage at postoperative 4 months (p > 0.05). However, there was a statistically significant difference in the functional success percentage in the triamcinolone-soaked group (92.0%) compared to the control group (78.3%; p = 0.035). When comparing postoperative complications, the triamcinolone-soaked group (4.0%) had a lower incidence of granulation than the control group (16.0%) (p = 0.032), but there were no differences in synechiae and membrane formation (p > 0.05). There was a statistically significant difference in the revision percentage in the triamcinolone-soaked group (4.0%) compared to the control group (16.0%) (p = 0.032).
REFERENCES
1). McDonogh M, Meiring JH. Endoscopic transnasal dacryocystorhinostomy. J Laryngol Otol. 1989; 103:585–7.

2). Park JW, Park HY, Yoon KC. Clinical effect of the mixed solution of sodium hyaluronate and sodium carboxymethylcellulose after endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 2010; 51:795–801.

3). Choi YJ, Hwang SJ, Lee TS. Short-term clinical results of amniotic membrane application to endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 2008; 49:384–9.

4). Kim JH, Shin JC. Clinical evaluation of endoscopic transnasal dacryocyocystorhinostomy. J Korean Ophthalmol Soc. 1997; 38:1706–11.
5). Metson R. Endoscopic surgery for lacrimal obstruction. Otolaryngol Head Neck Surg. 1991; 104:473–9.

6). Goldberg RA. Endonasal dacryocystorhinostomy: is it really less successful? Arch Ophthalmol. 2004; 122:108–10.
7). Mannor GE, Millman AL. The prognostic value of preoperative dacryocystography in endoscopic intranasal dacryocystorhinostomy. Am J Ophthalmol. 1992; 113:134–7.

8). Ali MJ, Wormald PJ, Psaltis AJ. The dacryocystorhinostomy ostium granulomas: classification, indications for treatment, management modalities and outcomes. Orbit. 2015; 34:146–51.

9). Li EY, Cheng AC, Wong AC, et al. Safety and efficacy of adjunctive intranasal mitomycin C and triamcinolone in endonasal endoscopic dacryocystorhinostomy. Int Ophthalmol. 2016; 36:105–10.

10). Sabarinath V, Harish MR, Divakaran S. Triamcinolone impregnated nasal pack in endoscopic sinus surgery: our experience. Indian J Otolaryngol Head Neck Surg. 2017; 69:88–92.

11). Côté DW, Wright ED. Triamcinolone-impregnated nasal dressing following endoscopic sinus surgery: a randomized, double-blind, placebo-controlled study. Laryngoscope. 2010; 120:1269–73.

12). Cheng AC, Wong AC, Sze AM, Yuen HK. Limited nasal septoplasty by ophthalmologists during endonasal dacryocystorhinostomy: is it safe? Ophthalmic Plast Reconstr Surg. 2009; 25:293–5.

13). Hellebrekers BW, Trimbos-Kemper TC, Trimbos JB, et al. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril. 2000; 74:203–12.

14). Lee TS, Kim SW, Park BW. The relationship between rate of wound healing and success rate after endonasal laser-drill assisted dacryocystorhinostomy. J Korean Ophthalmol Soc. 1999; 40:2969–74.
15). Leong M, Phillips LG. Wound healing. Townsend CM, editor. Sabiston Textbook of Surgery. 17th. Philadelphia: WB Saunders;2004. chap. 18.

16). Dolmetsch AM. Nonlaser endoscopic endonasal dacryocystorhinostomy with adjunctive mitomycin C in nasolacrimal duct obstruction in adults. Ophthalmology. 2010; 117:1037–40.

17). Leibovitch I, Prabhakaran VC, Davis G, Selva D. Intraorbital injection of triamcinolone acetonide in patients with idiopathic orbital inflammation. Arch Ophthalmol. 2007; 125:1647–51.

18). Ebner R, Devoto MH, Weil D, et al. Treatment of thyroid associated ophthalmopathy with periocular injections of triamcinolone. Br J Ophthalmol. 2004; 88:1380–6.

19). Zeldovich A, Ghabrial R. Revision endoscopic dacryocystorhinostomy with betamethasone injection underassisted local anaesthetic. Orbit. 2009; 28:328–31.
20). Xu J, Park SH, Park HS, et al. Effects of triamcinolone-impregnated nasal dressing on subjective and objective outcomes following endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2016; 273:4351–7.

21). Hong SD, Kim JH, Dhong HJ, et al. Systemic effects and safety of triamcinolone-impregnated nasal packing after endoscopic sinus surgery: a randomized, double-blinded, placebo-controlled study. Am J Rhinol Allergy. 2013; 27:407–10.

22). Kang IG, Yoon BK, Jung JH, et al. The effect of high-dose topical corticosteroid therapy on prevention of recurrent nasal polyps after revision endoscopic sinus surgery. Am J Rhinol. 2008; 22:497–501.

Figure 1.
Endoscopic view of one patient. Endoscopic appearance of the middle meatus (A) before endonasal dacryocystorhinostomy, (B) after endonasal dacryocystorhinostomy with triamcinolone-soaked Nasopore® packing.
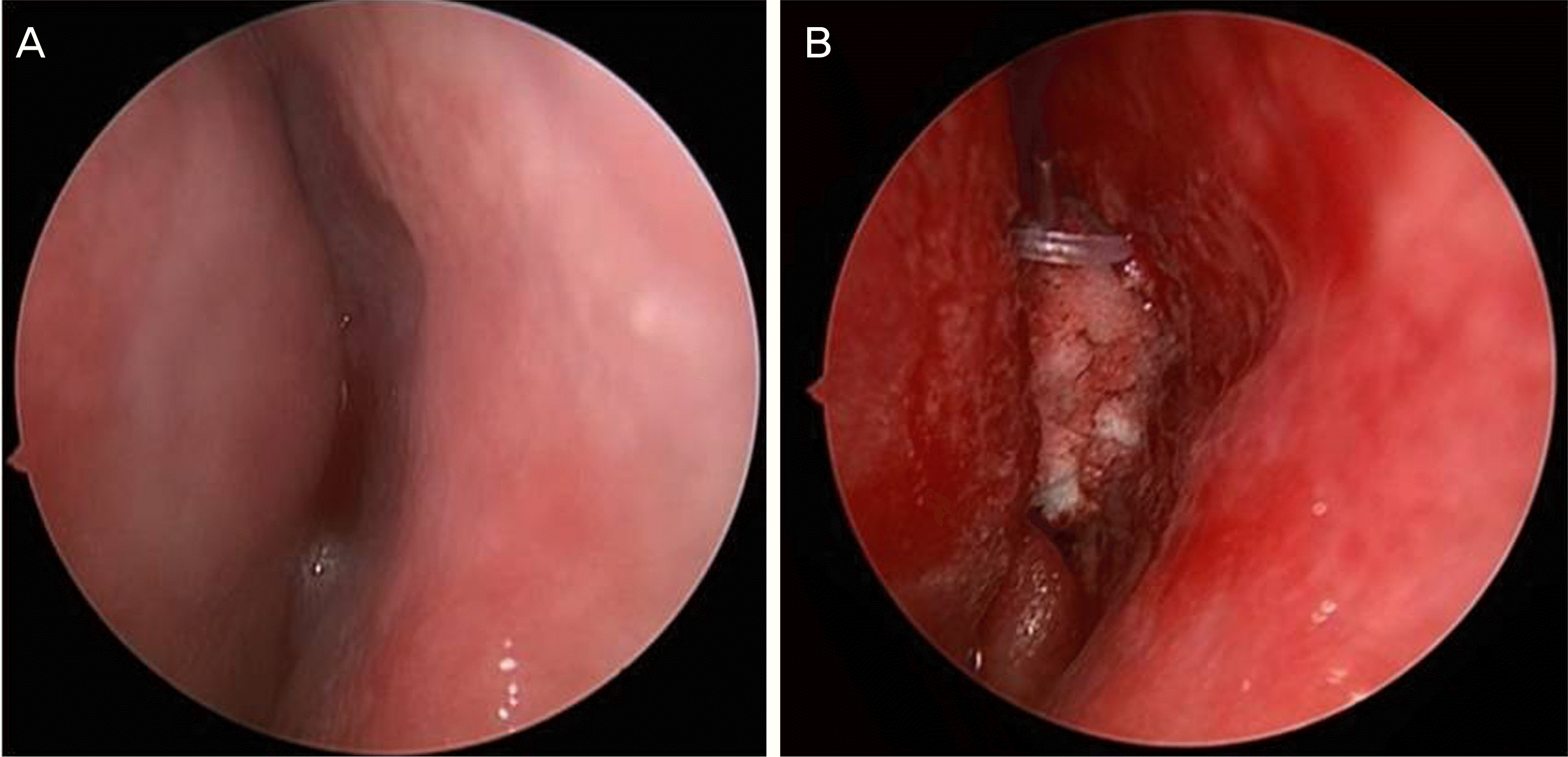
Figure 2.
Comparison of the anatomical success percentage between the two groups over time. At postoperative 2 months, there was a statistically significant difference in the anatomical success percentage in the triamcinolone-soaked group compared to the control group (p = 0.007). There were no statistically significant differences between the two groups in anatomical success percentage at postoperative 4 months (p > 0.05).
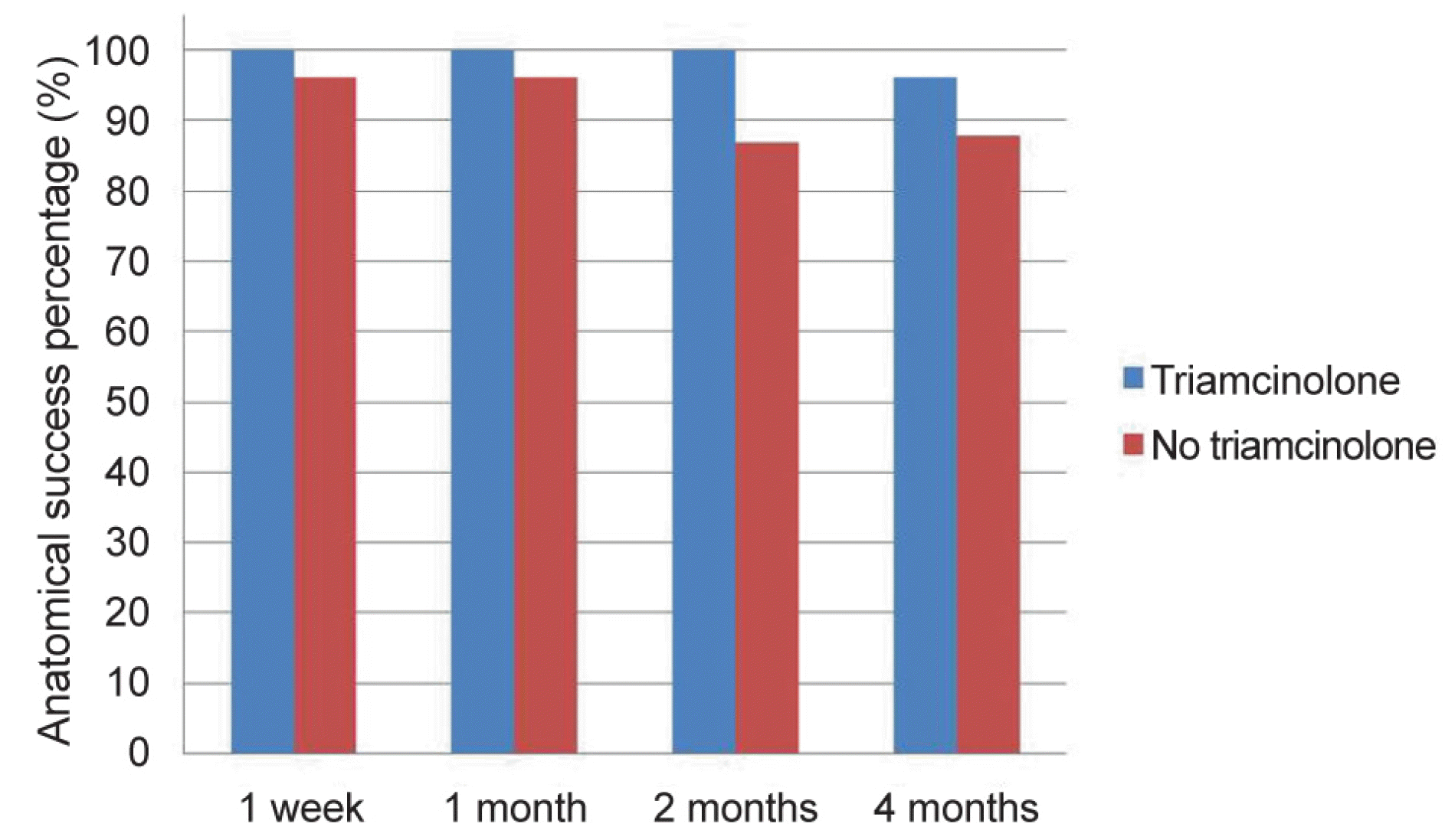
Figure 3.
Comparison of the functional success percentage between the two groups over time. At postoperative 4 months, there was a statistically significant difference in the functional success percentage between the two groups (p = 0.035).
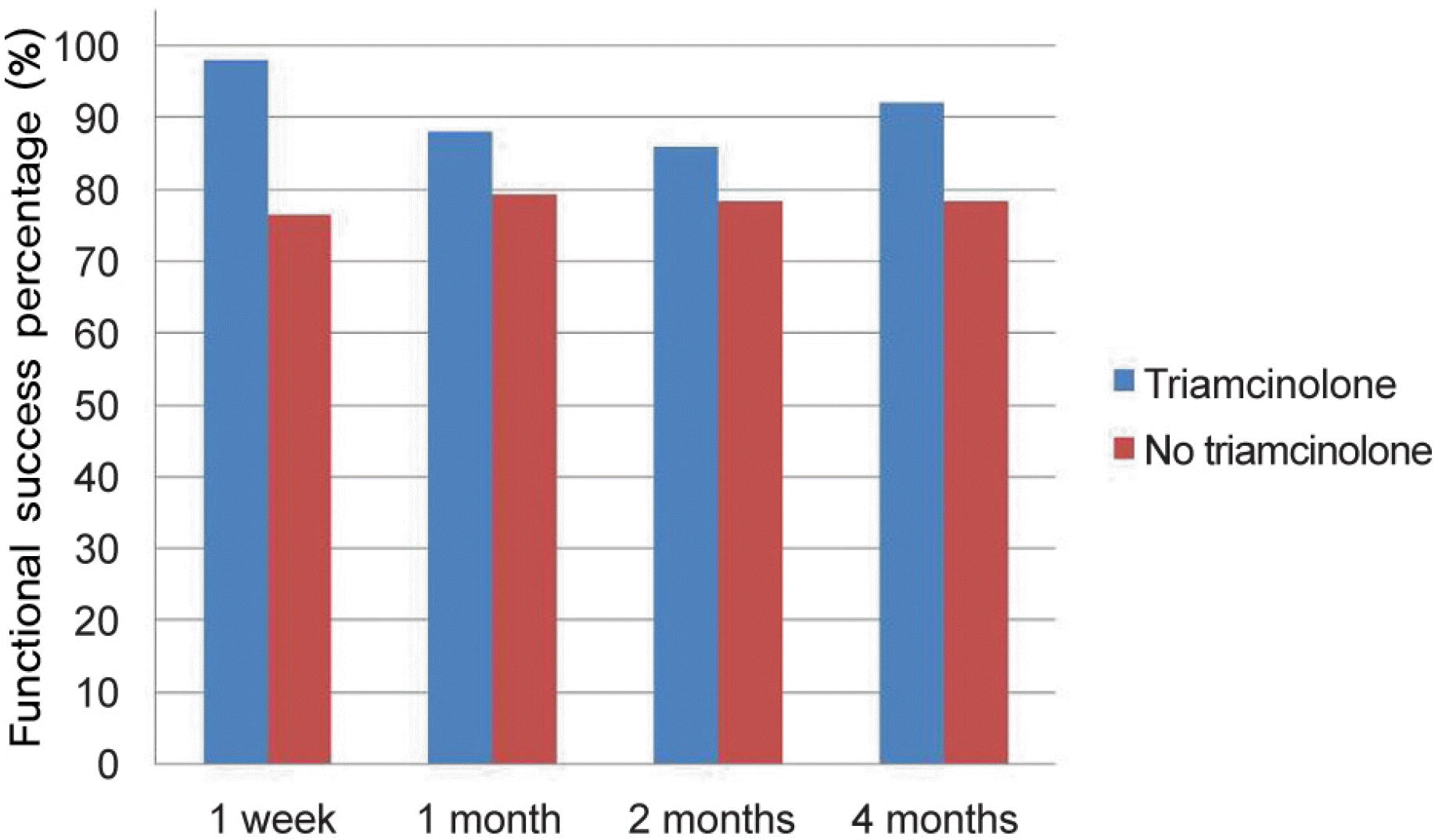
Table 1.
Patient demographics and characteristics at baseline
| No triamcinolone (n = 106) | Triamcinolone (n = 50) | p-value | |
|---|---|---|---|
| Age (years) | 59.74 ± 10.61 | 59.96 ± 13.47 | 0.165* |
| Sex (n, %) | |||
| Male | 21 (19.8) | 6 (12.0) | 0.229† |
| Female | 85 (80.2) | 44 (88.0) | |
| Side (n, %) | |||
| Right | 47 (44.3) | 28 (56.0) | 0.174† |
| Left | 59 (55.7) | 22 (44.0) | |
| DM (n, %) | 9 (84.9) | 1 (2.0) | 0.122† |
| HTN (n, %) | 28 (26.4) | 12 (24.0) | 0.747† |
| Antiplatelet (n, %) | 13 (12.3) | 3 (6.0) | 0.229† |
| Previous dacryocystitis history (n, %) | 3 (2.8) | 0 (0) | 0.230† |
| Mean time to tube removal (weeks) | 8.76 ± 2.24 | 8.80 ± 1.21 | 0.916* |
| Total follow up period (months) | 5.98 ± 1.79 | 6.08 ± 1.05 | 0.718* |
Table 2.
Comparison of the anatomical success percentage
| No triamcinolone (n = 106) | Triamcinolone (n = 50) | p-value* | |
|---|---|---|---|
| Postoperative 1 week (n, %) | 102 (96.2) | 50 (100) | 0.164 |
| Postoperative 1 month (n, %) | 102 (96.2) | 50 (100) | 0.164 |
| Postoperative 2 month (n, %) | 92 (86.8) | 50 (100) | 0.007 |
| Postoperative 4 month (n, %) | 93 (87.7) | 48 (96.0) | 0.102 |
Table 3.
Comparison of the functional success percentage
| No triamcinolone (n = 106) | Triamcinolone (n = 50) | p-value* | |
|---|---|---|---|
| Postoperative 1 week (n, %) | 81 (76.4) | 49 (98.0) | 0.001 |
| Postoperative 1 month (n, %) | 84 (79.2) | 44 (88.0) | 0.184 |
| Postoperative 2 month (n, %) | 83 (78.3) | 43 (86.0) | 0.255 |
| Postoperative 4 month (n, %) | 83 (78.3) | 46 (92.0) | 0.035 |
Table 4.
Comparison of postoperative complications
| No triamcinolone (n = 106) | Triamcinolone (n = 50) | p-value* | |
|---|---|---|---|
| Revision (n, %) | 17 (16.0) | 2 (4.0) | 0.032 |
| Granuloma formation (n, %) | 17 (16.0) | 2 (4.0) | 0.032 |
| Synechiae (n, %) | 2 (1.8) | 1 (2.0) | 0.962 |
| Membrane formation (n, %) | 5 (4.7) | 0 (0) | 0.119 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download