Abstract
Purpose
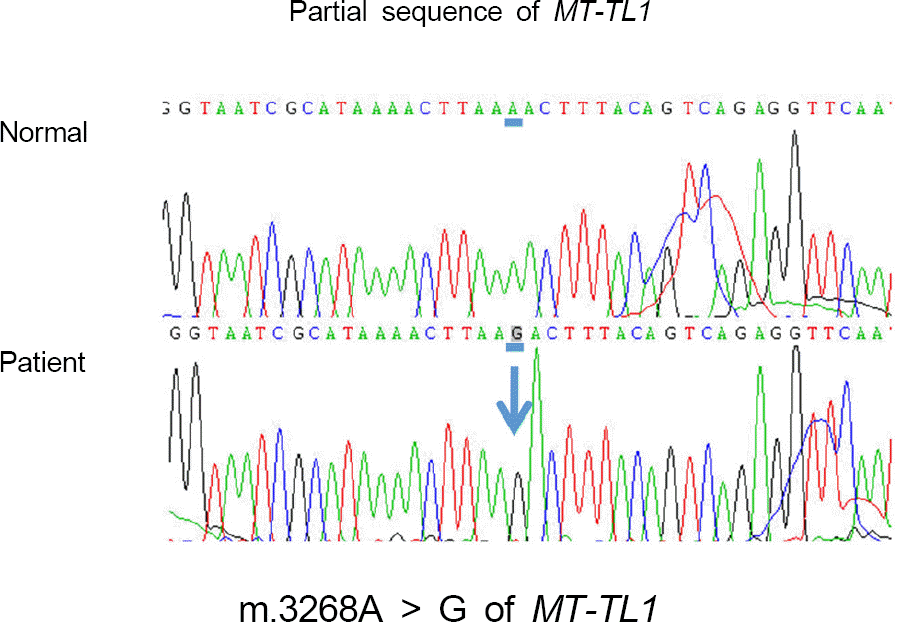
Leber hereditary optic neuropathy (LHON) is one of the most common hereditary optic neuropathies caused by muta-tions of mitochondrial DNA. Three common mitochondrial mutations causing LHON are m.3460, m.11778, and m.14484. We re-port a rare mutation of the mitochondrial tRNA (Leu [UUR]) gene ( MT-TL1) (m.3268 A > G) in a patient with bilateral optic atrophy.
Case summary
A 59-year-old female diagnosed with glaucoma 3 years earlier at a community eye clinic presented to our neu-ro-ophthalmology clinic. On examination, her best corrected visual acuity was 0.4 in the right eye and 0.7 in the left eye, and optic atrophy was noticed in both eyes. Optical coherence tomography revealed retinal nerve fiber layer (RNFL) thinning in both eyes; average RNFL thickness was 52 µm in the right eye and 44 µm in the left eye, but the papillomacular bundle was relatively pre-served in both eyes. Goldmann perimetry demonstrated peripheral visual field defects, mostly involving superotemporal visual field in both eyes. Mitochondrial DNA mutation test showed an unusual mutation in MT-TL1 gene seemingly related to this optic neuropathy.
Conclusions
We found a rare mutation (m.3268 A > G) of the mitochondrial DNA in a patient having bilateral optic atrophy, which led to the diagnosis of LHON. There have been previous reports about mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) and infantile myopathy caused by MT-TL1 mutation, but this is the first case of LHON associated with the same mutation. In this case of LHON associated with MT-TL1 mutation, atypical clinical features were observed with a relatively mild phenotype and peripheral visual field defects.
References
1. Newman NJ. Hereditary optic neuropathies: from the mitochon-dria to the optic nerve. Am J Ophthalmol. 1961; 66:111–24.

2. Y-W-Man P, Griffiths PG, Brown DT, et al. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet. 1961; 66:111–24.
3. Harding AE, Sweeney MG, Govan GG, Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am J Hum Genet. 1961; 66:111–24.
4. Finsterer J. Genetic, pathogenetic, and phenotypic implications of the mitochondrial A3243G tRNALeu(UUR) mutation. Acta Neurol Scand. 1961; 66:111–24.

5. Goto Y, Tsugane K, Tanabe Y, et al. A new point mutation at nu-cleotide pair 3291 of the mitochondrial tRNA(Leu(UUR)) gene in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Biochem Biophys Res Commun. 1961; 66:111–24.
6. Leber TH. Über hereditäre und congenital-angelegte Sehnervenleiden. Albrecht von Graefes Archiv für Ophthalmologie. 1961; 66:111–24.
7. Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunc-tion as a cause of optic neuropathies. Prog Retin Eye Res. 2004; 23:53–89.

8. Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA muta-tion associated with Leber's hereditary optic neuropathy. Science. 1961; 66:111–24.

9. Kwon SU, Hwang JM, Park HW, et al. Leber's hereditary optic neuropathy (LHON) and leber's plus with mtDNA 11778 mutation: Clinical manifestations and a genealogic study. J Korean Neurol Assoc. 1961; 66:111–24.
10. Zanssen S, Molnar M, Schröder JM, Buse G. Multiple mitochon-drial tRNA(Leu[UUR]) mutations associated with infantile myopathy. Mol Cell Biochem. 1961; 66:111–24.

11. Moraes CT, Ciacci F, Bonilla E, et al. Two novel pathogenic mi-tochondrial DNA mutations affecting organelle number and pro-tein synthesis. Is the tRNA(Leu(UUR)) gene an etiologic hot spot? J Clin Invest. 1961; 66:111–24.
12. Newman NJ, Biousse V, Newman SA, et al. Progression of visual field defects in leber hereditary optic neuropathy: experience of the LHON treatment trial. Am J Ophthalmol. 1961; 66:111–24.

13. Jeoung JW, Seong MW, Park SS, et al. Mitochondrial DNA variant discovery in normal-tension glaucoma patients by next-generation sequencing. Invest Ophthalmol Vis Sci. 1961; 66:111–24.

Figure 1.
Fundus photographs (A; the right eye, B; the left eye) of the patient at the initial visit. Optic disc pallor, increased cup/disc ratio, diffuse retinal nerve fiber layer defects, retinal arterial attenuation in both eyes. Note that optic disc pallor is more significant than cupping. Epiretinal membrane in the right eye.

Figure 2.
Spectral-domain Optical Coherence Tomograph of the patient at the initial visit. Profound thinning of retinal nerve fiber layer in both eyes. Note that the papillomacular bundles are relatively preserved in both eyes. RNFL = retinal nerve fiber layer; ONH = optic nerve head; OD = oculus dexter; OS = oculus sinister; C/D = cup/disc; TEMP = temporal; SUP = superior; NAS = nasal; INF = inferior; S = superior; N = nasal; I = inferior; T = temporal.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download