Abstract
Purpose
To compare the efficacy of intravitreal dexamethasone implant according to previous responses to Bevacizumab treatment in patients with macular edema (ME) secondary to branch retinal vein occlusion (BRVO).
Methods
Sixty eyes of 60 patients who received an intravitreal dexamethasone implant for ME secondary to BRVO and followed up for at least 6 months were retrospectively reviewed. Of these, 31 patients were treatment naïve and 29 patients had previously received intravitreal injection of anti-vascular endothelial growth factor (VEGF). Out of these previously-treated patients, 17 patients were categorized as a refractory group who did not respond to previous injection and 12 patients were categorized as a responder group who showed recurrent ME despite a good response to previous anti-VEGF treatment. The best corrected visual acuity (BCVA), central macular thickness (CMT) and recurrence of ME were assessed monthly for 6 months.
Results
At each 3-month follow-up, the BCVA improved significantly from baseline in the naïve group, while the refractory group and the responder group showed significant improvement for only 2 months. At each 3-month follow-up, the CMT showed significant decreases in every group. However, the mean change in CMT from baseline showed significant differences between the 3 groups at month 3 (p < 0.001). During follow-up, 18 eyes in the naïve group (58.1%), 16 eyes in the refractory group (94.1%), and 6 eyes in the responder group (50.0%) received retreatment for the recurrence of ME, and there was a significant difference in the retreatment rate between the three groups (p = 0.016).
Conclusions
Intravitreal dexamethasone implant showed early good functional and anatomical improvements irrespective of the response to the previous treatment in patients with ME secondary to BRVO. However, when treating the refractory group, more careful observation and intensive retreatment are required, considering the short duration of its efficacy.
References
1. Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical abdominals, diagnosis, and complications. An update of the literature. Retina. 2013; 33:901–10.
2. Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of abdominall vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000; 98:133–41. discussion 141–3.
3. Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008; 33:111–31.

4. Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for abdominal edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008; 16:791–9.
5. Pe'er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998; 105:412–6.
6. Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997; 12:99–109.
7. Antonetti DA, Barber AJ, Hollinger LA, et al. Vascular endothelial growth factor induces rapid phosphorylation of tight junction abdominal occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999; 274:23463–7.
8. Antonetti DA, Barber AJ, Khin S, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial abdominal content: vascular endothelial growth factor decreases abdominal in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998; 47:1953–9.
9. Suzuki Y, Nakazawa M, Suzuki K, et al. Expression profiles of abdominal and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol. 2011; 55:256–63.
10. Guigou S, Hajjar C, Parrat E, et al. Multicenter Ozurdex(R) abdominal for diabetic macular edema: MOZART study. J Fr Ophtalmol. 2014; 37:480–5.
11. Escobar-Barranco JJ, Pina-Marín B, Fernández-Bonet M. Dexamethasone implants in patients with naïve or refractory diffuse abdominal macular edema. Ophthalmologica. 2015; 233:176–85.
12. Sharareh B, Gallemore R, Taban M, et al. Recalcitrant macular abdominal after intravitreal bevacizumab is responsive to an intravitreal dexamethasone implant in retinal vein occlusion. Retina. 2013; 33:1227–31.
13. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-abdominalled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.e3.
14. Sheu SJ, Wu TT, Horng YH. Efficacy and safety of dexamethasone intravitreal implant for treatment of refractory macular edema abdominal to retinal vein occlusion in Taiwan. J Ocul Pharmacol Ther. 2015; 31:461–7.
15. Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: abdominal or tolerance? Br J Ophthalmol. 2012; 96:1–2.
16. Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to abdominal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology. 2008; 115:2199–205.
17. Shahsuvaryan ML. Therapeutic potential of intravitreal abdominal in retinal vein occlusion. Int J Ophthalmol. 2012; 5:759–70.
18. Ip MS, Gottlieb JL, Kahana A, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol. 2004; 122:1131–6.
19. Ozdek SC, Aydin B, Gürelik G, et al. Effects of intravitreal abdominal injection on macular edema and visual prognosis in central retinal vein occlusion. Int Ophthalmol. 2005; 26:27–34.
20. Wessel MM, Nair N, Aaker GD, et al. Peripheral retinal ischaemia, as evaluated by abdominalfield fluorescein angiography, is abdominal with diabetic macular oedema. Br J Ophthalmol. 2012; 96:694–8.
Figure 1.
Mean change in BCVA. (A) Mean BCVA during the follow-up period. (B) Mean change in BCVA from baseline during the follow-up period. At each visit of 6-month follow-up, the BCVA improved significantly from baseline in the naïve group, while the refractory group and the responder group showed significant improvement for only 2 months.
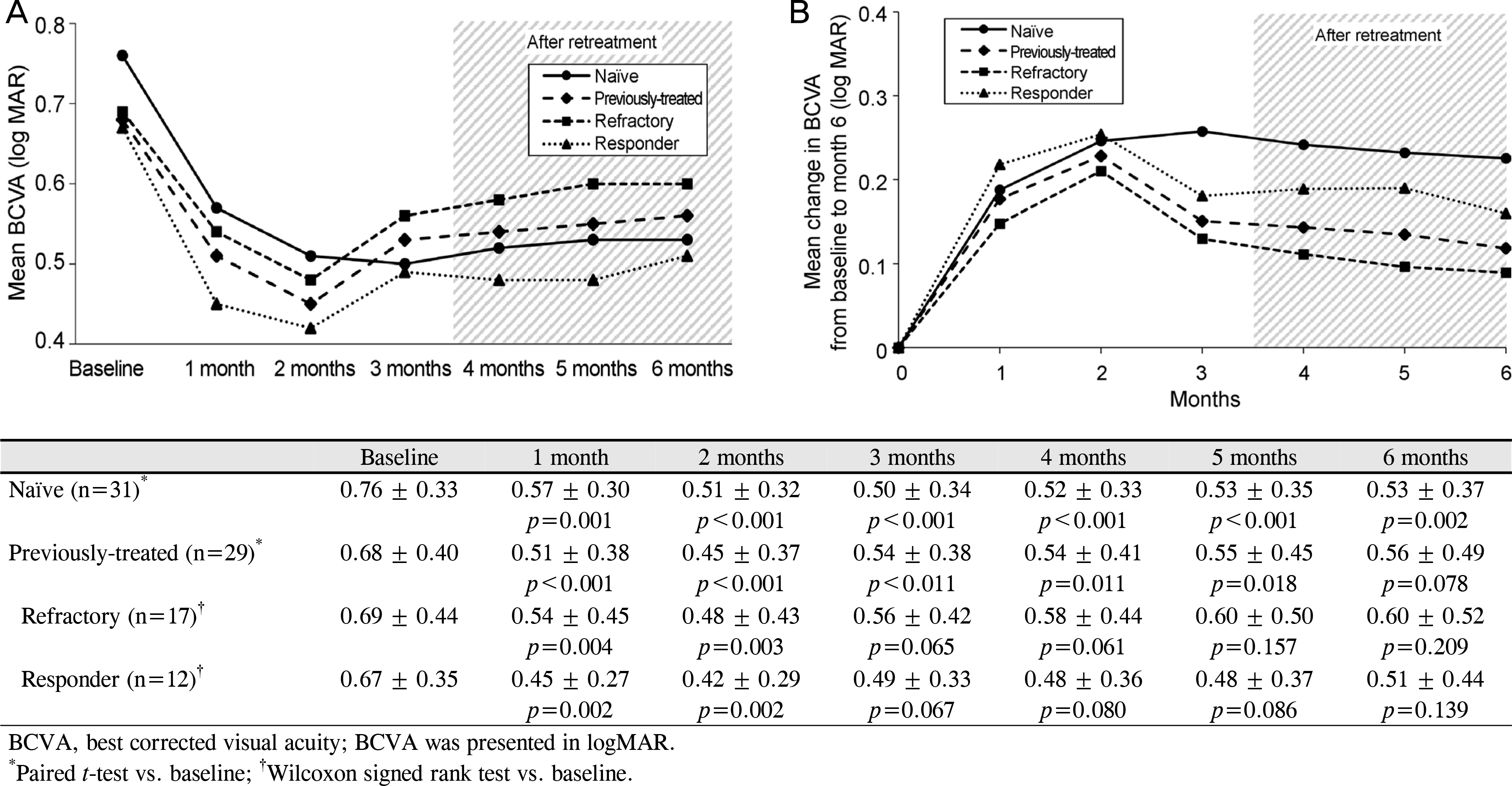
Figure 2.
Mean change in CMT. (A) Mean CMT during the follow-up period. (B) Mean change in CMT from baseline during the follow-up period. The CMT showed significant decrease at each visit in the naïve group and the responder group, and the refractory group showed decrease in CMT for only 3 months. There were significant differences between the groups at month 3, 4, 5 and 6 (p < 0.001 at month 3, 4 and 5, p = 0.013 at month 6).
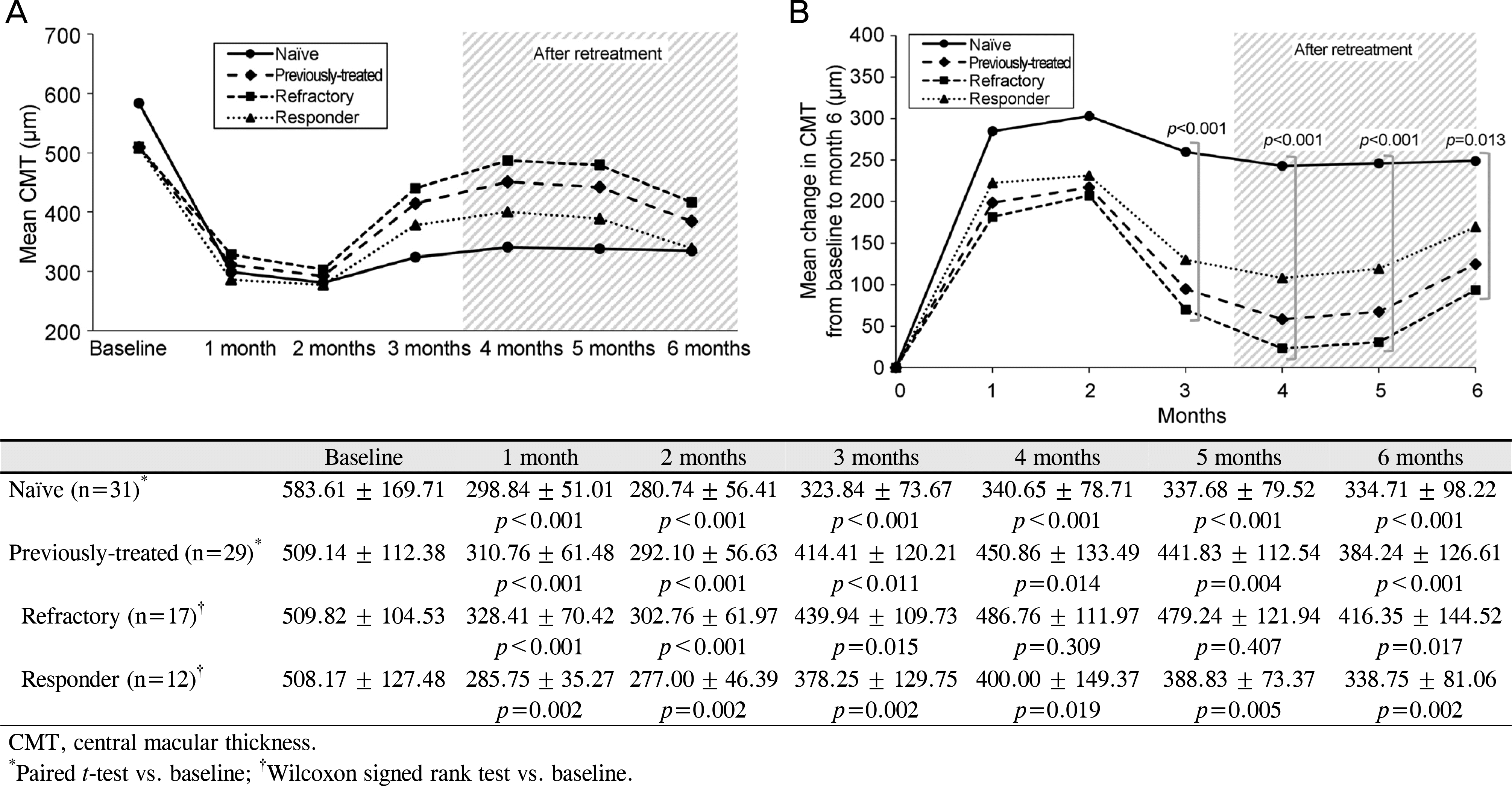
Table 1.
Demographic and baseline characteristics of patients
| Naïve | Previously-treated | p-value |
Previously-treated |
p-value | ||
|---|---|---|---|---|---|---|
| Refractory | Responder | |||||
| Number of eyes | 31 | 29 | 17 | 12 | ||
| Gender | ||||||
| Male | 12 | 8 | 0.361* | 5 | 3 | 0.793* |
| Female | 19 | 21 | 0.361 | 12 | 9 | 0.793 |
| OD/OS | 18/13 | 19/10 | 0.553* | 12/5 | 7/5 | 0.494* |
| Age | 64.77 ± 10.55 | 62.59 ± 7.73 | 0.366† | 63.35 ± 7.27 | 61.50 ± 8.53 | 0.328† |
| Diabetes mellitus (n, %) | 4 (12.9) | 3 (10.3) | 0.538‡ | 3 (17.6) | 0 (0.0) | 0.246‡ |
| Hypertension (n, %) | 14 (45.2) | 8 (27.6) | 0.158* | 4 (23.5) | 4 (33.3) | 0.683‡ |
| Lens status (phakic/pseudophakic) | 25/6 | 27/2 | 0.257‡ | 15/2 | 12/0 | 0.498‡ |
| Occlusion site (major branch/macular b | branch) 20/11 | 21/8 | 0.511* | 12/5 | 9/3 | 0.793* |
| Macular ischemia (n, %) | 13 (41.9) | 14 (48.3) | 0.622* | 11 (64.7) | 3 (25.0) | 0.035* |
| Peripheral ischemia (n, %) | 12 (38.7) | 19 (65.5) | 0.038* | 14 (82.4) | 5 (41.7) | 0.023* |
| Baseline BCVA (log MAR) | 0.76 ± 0.33 | 0.68 ± 0.40 | 0.430† | 0.69 ± 0.44 | 0.59 ± 0.37 | 0.446† |
| Baseline CMT (μ m) | 583.61 ± 169.71 | 509.14 ± 112.38 | 0.051†5 | 509.82 ± 104.53 | 508.17 ± 127.48 | 0.879† |
Table 2.
Number of patients of retreatment in each group
| Naïve |
Previously-treated |
p-value* | ||
|---|---|---|---|---|
| Refractory | Responder | |||
| Retreatment | 18 (58.1) | 16 (94.1) | 6 (50.0) | 0.016 |
| No retreatment | 13 (41.9) | 1 (5.9) | 6 (50.0) | |
| Total | 31 (100.0) | 17 (100.0) | 12 (100.0) | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download