Abstract
Purpose
Methods
Results
References
Figure 1.
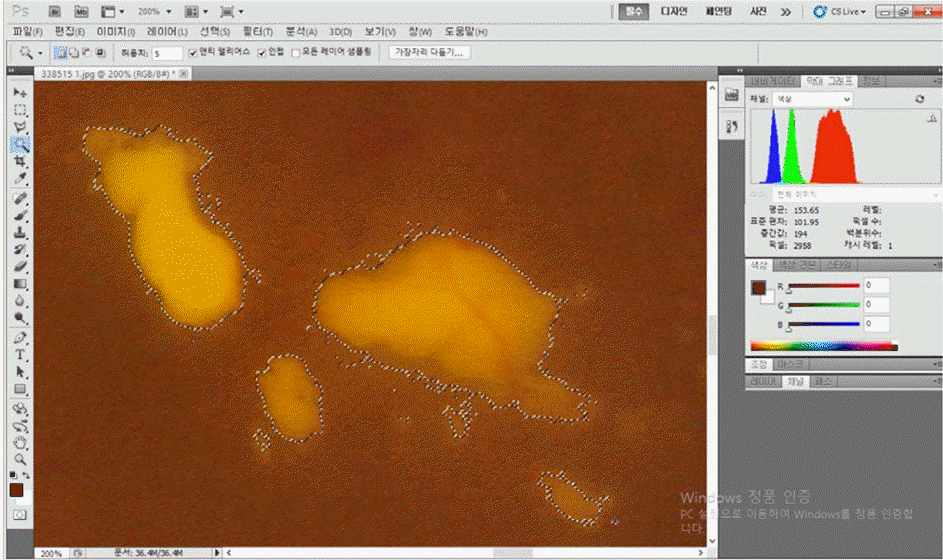
Figure 2.
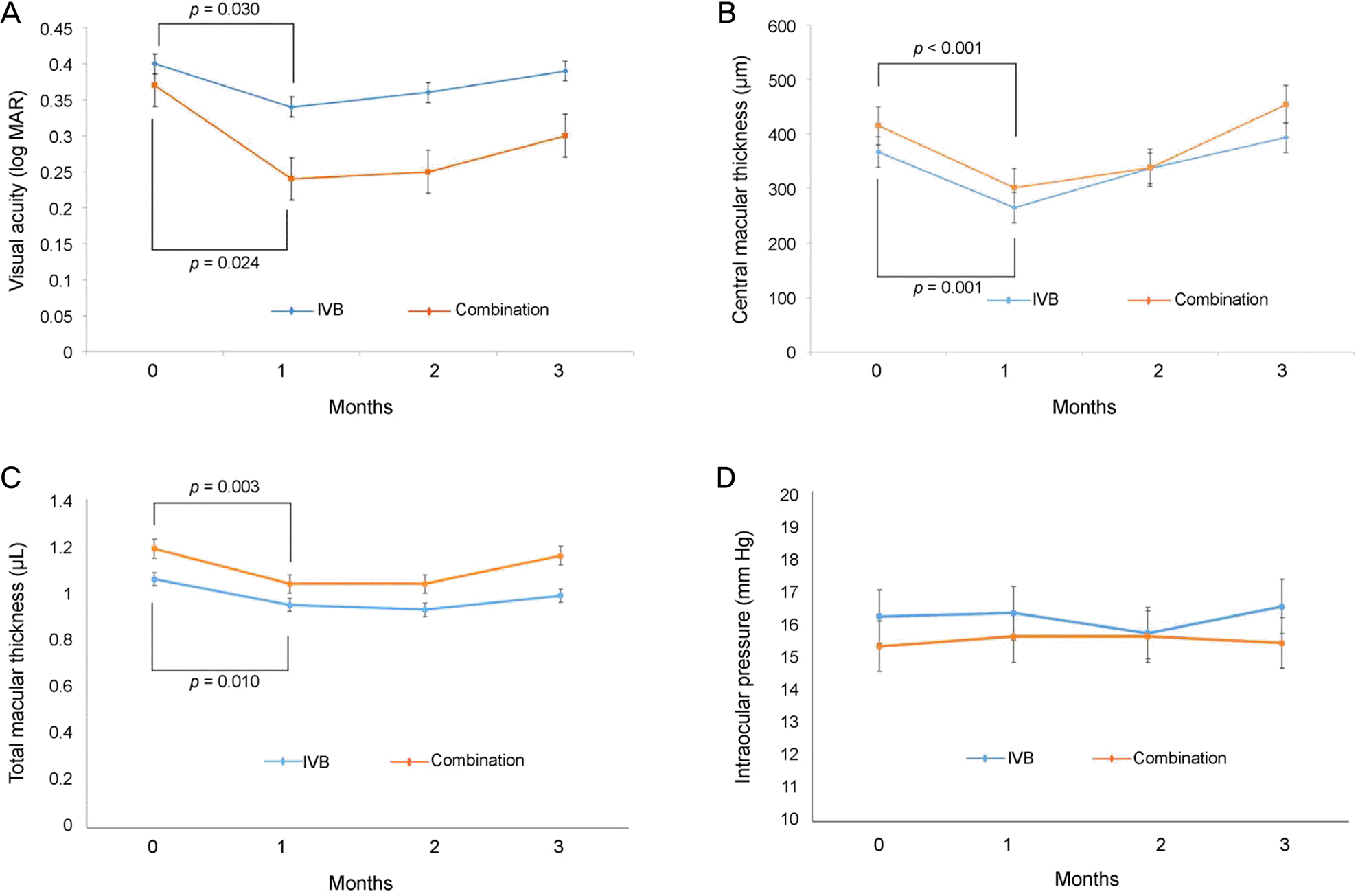
Figure 3.
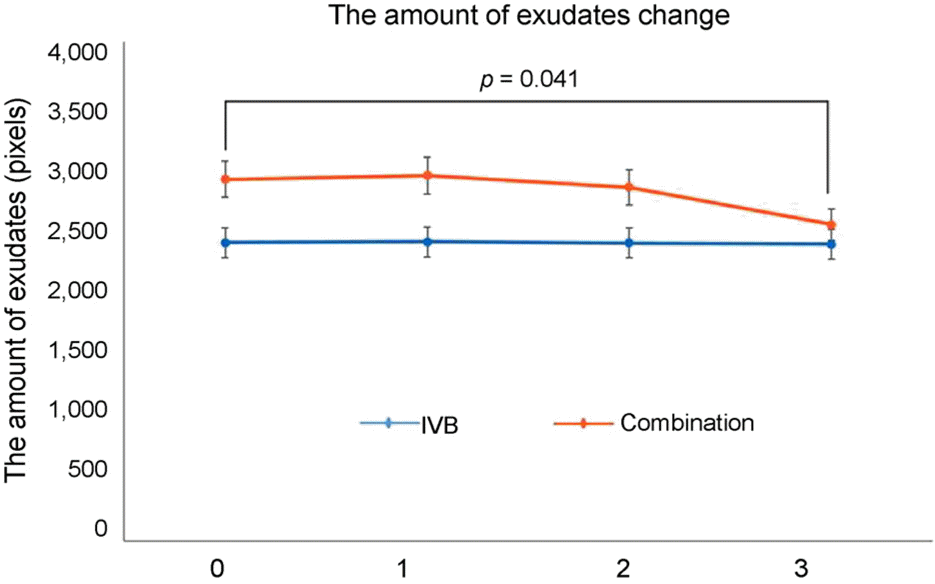
Table 1.
| IVB group | Combination group | p-value | |
|---|---|---|---|
| Eyes | 35 | 31 | |
| Age (years) | 53.9 ± 9.1 | 51.7 ± 7.9 | 0.275* |
| Male:Female | 27:8 | 18:13 | 0.058† |
| Right:Left | 17:18 | 14:17 | 0.807† |
| Duration of diabetes (years) | 9.3 ± 7.2 | 7.11 ± 6.2 | 0.316* |
| Hypertension (yes:no) | 18:17 | 10:21 | 0.082* |
| Visual acuity (log MAR) | 0.40 ± 0.27 | 0.37 ± 0.19 | 0.091* |
| Intraocular pressure (mm Hg) | 17.5 ± 3.6 | 15.7 ± 3.7 | 0.124* |
| Central macular thickness (μ m) | 367.0 ± 113.6 | 415.9 ± 157.8 | 0.262* |
| Total macular volume (mm3) | 1.06 ± 0.08 | 1.19 ± 0.04 | 0.165* |
| Amount of hard exudates (pixels) | 2,394 ± 1,989 | 2899 ± 2,314 | 0.235* |
Values are presented as mean ± SD unless otherwise indicated. IVB group = intravitreal bevacizumab injections in diabetic macular edema; Combination group = combined therapy with intravitreal bevacizumab and subtenon triamcinolone acetonide injection in diabetic macular edema.
Table 2.
|
IVB grou |
Combination group |
|||||
|---|---|---|---|---|---|---|
| Mean ± SD | p-value* | Mean ± SD | p-value† | p-value‡ | ||
| Visual acuity (log MAR) | At baseline | 0.40 ± 0.27 | 0.37 ± 0.19 | 0.291 | ||
| 1 month | 0.34 ± 0.22 | 0.030 | 0.24 ± 0.22 | 0.024 | 0.124 | |
| 2 months | 0.36 ± 0.23 | 0.916 | 0.25 ± 0.19 | 0.306 | 0.077 | |
| 3 months | 0.39 ± 0.28 | 0.854 | 0.30 ± 0.21 | 0.285 | 0.153 | |
| Central macular thickness (μm) | At baseline | 367.0 ± 113 | 415.9 ± 157 | 0.262 | ||
| 1 month | 265 ± 104 | <0.001 | 302 ± 144 | 0.001 | 0.223 | |
| 2 months | 337 ± 129 | 0.196 | 338 ± 194 | 0.066 | 0.982 | |
| 3 months | 394 ± 130 | 0.410 | 454 ± 221 | 0.953 | 0.344 | |
| Total macular volume (mm3) | At baseline | 1.06 ± 0.08 | 1.19 ± 0.04 | 0.165 | ||
| 1 month | 0.95 ± 0.09 | 0.003 | 1.04 ± 0.10 | 0.010 | 0.211 | |
| 2 months | 0.93 ± 0.10 | 0.059 | 1.04 ± 0.12 | 0.068 | 0.351 | |
| 3 months | 0.99 ± 0.14 | 0.790 | 1.15 ± 0.09 | 0.590 | 0.245 | |
| Amount of hard exudates (pixels) | At baseline | 2,394 ± 1,989 | 2,899 ± 2,314 | 0.235 | ||
| 1 month | 2,400 ± 1,998 | 0.871 | 2,930 ± 2,015 | 0.865 | 0.278 | |
| 2 months | 2,389 ± 1,994 | 0.899 | 2,837 ± 2,020 | 0.217 | 0.343 | |
| 3 months | 2,379 ± 2,030 | 0.968 | 2,536 ± 1,981 | 0.041 | 0.608 | |
| Intraocular pressure (mm Hg) | At baseline | 16.2 ± 3.5 | 15.3 ± 2.8 | 0.164 | ||
| 1 month | 16.3 ± 4.1 | 0.879 | 15.6 ± 3.2 | 0.812 | 0.491 | |
| 2 months | 15.7 ± 3.9 | 0.535 | 15.6 ± 2.9 | 0.815 | 0.172 | |
| 3 months | 16.5 ± 4.4 | 0.689 | 15.4 ± 3.3 | 0.977 | 0.173 | |
Values are presented as mean ± SD unless otherwise indicated. IVB group = intravitreal bevacizumab injections in diabetic macular edema; Combination group = combined therapy with intravitreal bevacizumab and subtenon triamcinolone acetonide injection in diabetic macular edema.
* p-value of values at each follow-up period comparing to baseline values in intravitreal bevacizumab injection group, Wilcoxon matched-pairs signed ranks test




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download