Abstract
Purpose
Charcot-Marie-Tooth disease type 2A (CMT2A) is caused by mutations in the mitofusin 2 (MFN2) genes associated with variable central nervous system (CNS) involvement. The authors report a case of a middle-aged woman with genetically confirmed CMT type 2 (CMT2), combined with delayed-onset bilateral optic neuropathy.
Case summary
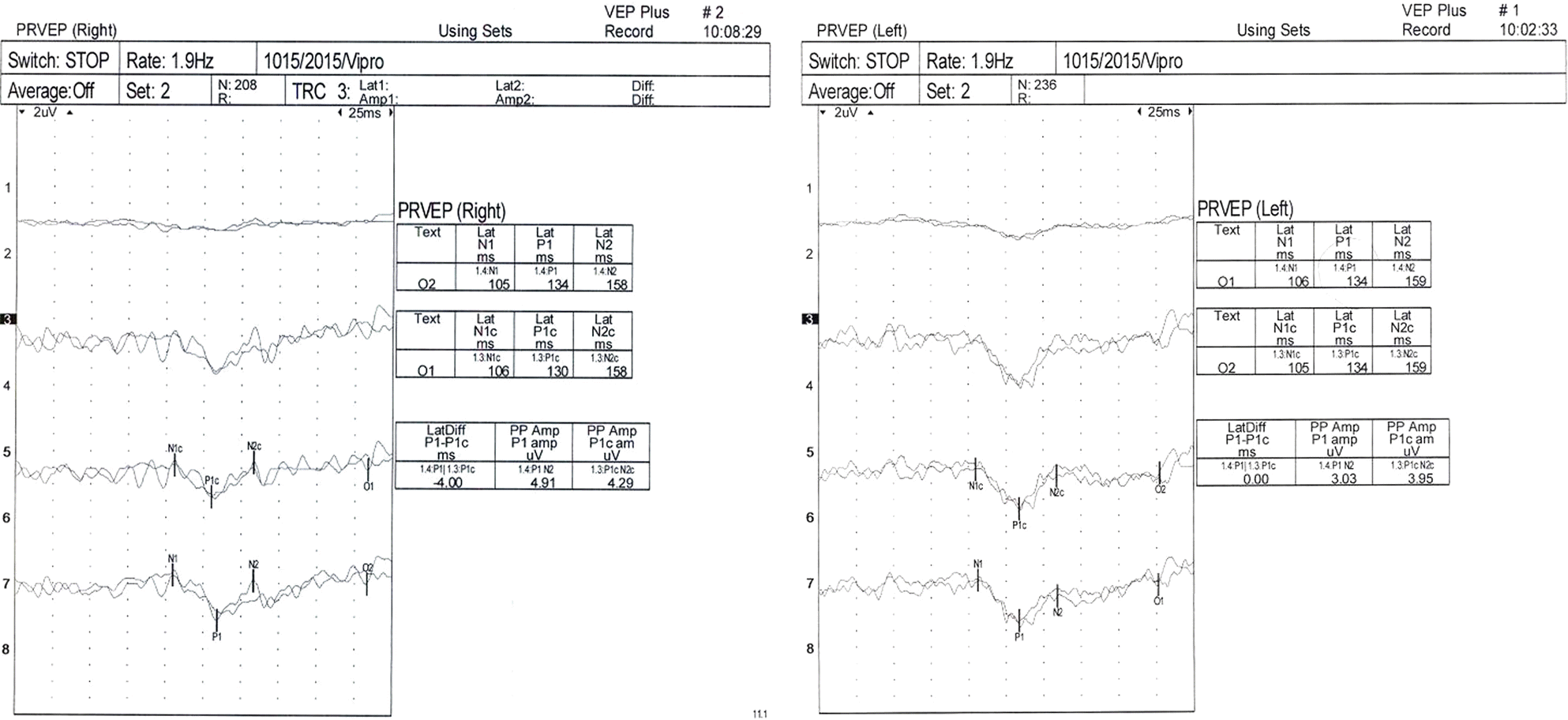
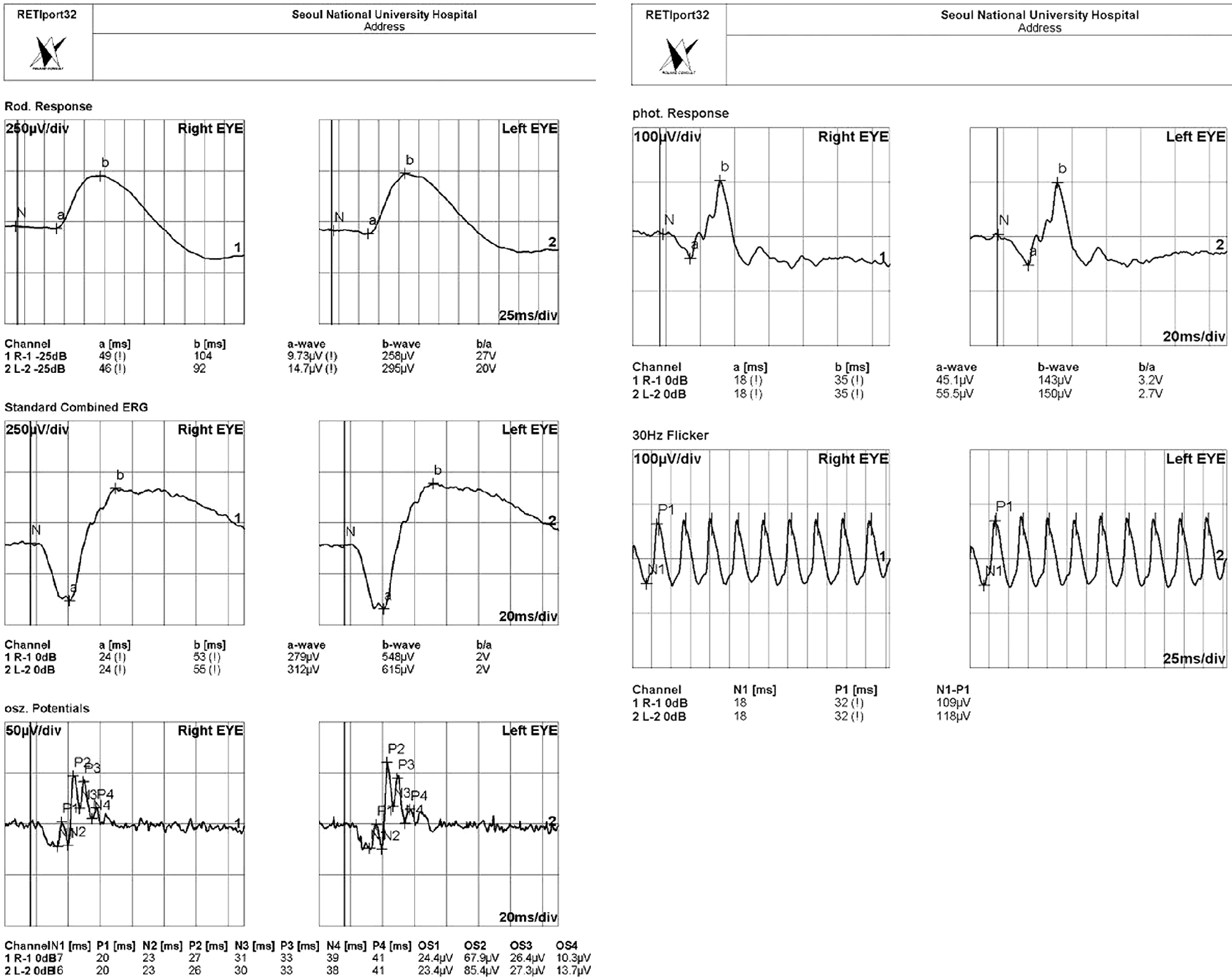
A 47-year-old woman presented with complaints of subacute decrease of visual acuity in both eyes. Her corrected visual acuity was 20/200 in the right eye and 20/320 in the left eye. Fundus photographs revealed bilateral disc pallor and diffuse retinal nerve fiber layer defects. No papillomacular bundle defect was observed. Goldmann perimetry showed central scotoma in both eyes. She had suffered from muscle wasting of the legs and foot deformities such as high arches and hammer toes since childhood and required a wheelchair for ambulation. A series of CMT gene mutation tests revealed an MFN2 gene mutation, c.617C>T (p.Thr206Ile), and the patient was diagnosed with CMT2A.
Conclusions
Charcot-Marie-Tooth disease is a common inherited neuromuscular disorder and CMT2A, an axonal CMT neuropathy, is associated with bilateral optic neuropathy. Therefore, suspecting CMT and testing for gene mutations as part of the work-up in patients with subacute bilateral optic neuropathy associated with peripheral neuropathy is critical.
References
1. Voo I, Allf BE, Udar N, et al. Hereditary motor and sensory neuropathy type VI with optic atrophy. Am J Ophthalmol. 2003; 136:670–7.

3. Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin Genet. 1974; 6:98–118.

4. Pareyson D, Marchesi C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 2009; 8:654–67.

5. Barisic N, Claeys KG, Sirotkovic-Skerlev M, et al. Charcot-Marie-Tooth disease: a clinico-genetic confrontation. Ann Hum Genet. 2008; 72:416–41.

6. Zühner S, De Jonghe P, Jordanova A, et al. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol. 2006; 59:276–81.
7. Chung KW, Kim SB, Park KD, et al. Early onset severe and late-onset mild Charcot-Marie-Tooth disease with mitofusin 2 (MFN2) mutations. Brain. 2006; 129:2103–18.

8. Wakerley BR, Harman FE, Altmann DM, Malik O. Charcot-Marie-Tooth disease associated with recurrent optic neuritis. J Clin Neurosci. 2011; 18:1422–3.

9. Kim HJ, Sohn KM, Shy ME, et al. Mutations in PRPS1, which enc-odes the phosphoribosyl pyrophosphate synthetase enzyme critical for nucleotide biosynthesis, cause hereditary peripheral neuropathy with hearing loss and optic neuropathy (cmtx5). Am J Hum Genet. 2007; 81:552–8.

10. Sadun AA, Win PH, Ross-Cisneros FN, et al. Leber's hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc. 2000; 98:223–32.
Figure 1.
Color fundus photograph of right eye (A) and color fundus photograph for left eye (B) of the patient at the initial visit. Disc pallor is observed but disc hyperemia or pseudoedema is not observed in both eyes. Note and margin of the retinal nerve fiber layer defect is not definite in both eyes.
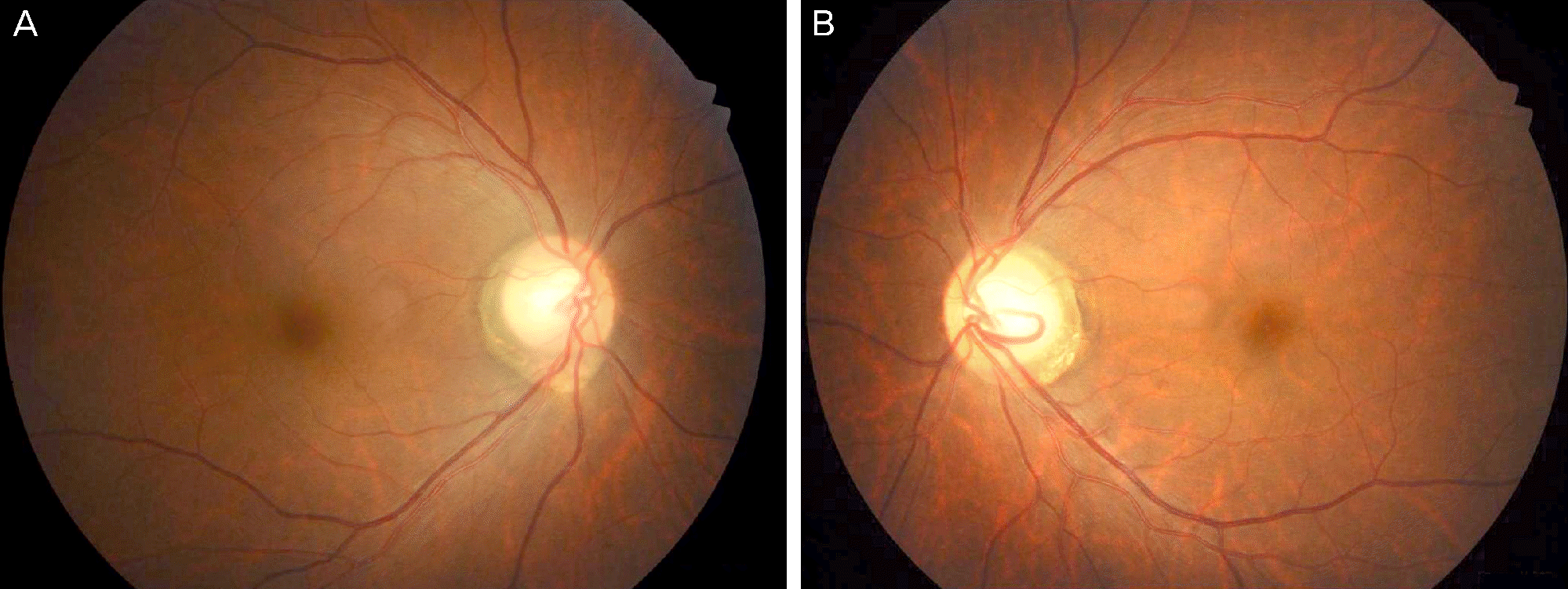
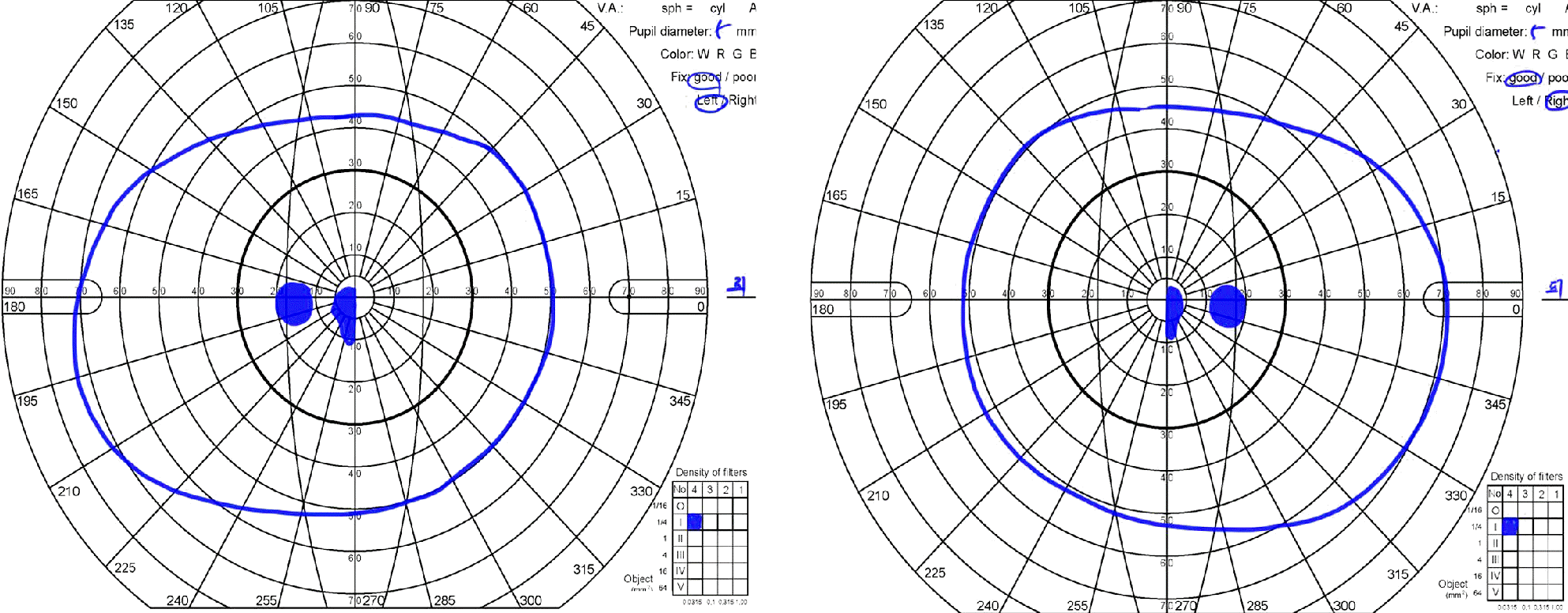
Figure 2.
Goldmann visual fields (GVF) reveal central scotoma in both eyes. She had suffered a simultaneous subacute visual acuity decrease in her both eyes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download