Abstract
Purpose
To compare clinical outcomes between intravitreal bevacizumab (IVB) monotherapy and combined therapy with half-fluence rate verteporfin photodynamic therapy (PDT) for occult choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD).
Methods
Medical records were reviewed in consecutive patients who underwent IVB monotherapy or combined therapy with PDT for occult CNV secondary to AMD and had a 12-month follow-up period. After 3 consecutive monthly IVB injections, both groups were eligible for additional IVB injections when necessary. Best-corrected visual acuity (BCVA), central macular thickness (CMT), and number of additional IVB injections were compared between the groups.
Results
Thirty-nine eyes underwent IVB monotherapy (IVB group) and 25 eyes underwent combined therapy (PDT+IVB group). Mean BCVA improved significantly in the PDT+IVB group (p = 0.046) and not in IVB group (p = 0.213). A significant reduction in mean CMT occurred in both groups (p< 0.001). The mean number of additional IVB injections was 1.6 ± 1.33 in the IVB group and 0.5 ± 1.01 in the PDT+IVB group (p = 0.001). There were no serious complications.
References
1. Verteporfin Roundtable Participants. Guidelines for using verte- porfin (Visudyne) in photodynamic therapy for choroidal neovascularization due to age-related macular degeneration and other causes: update. Retina. 2005; 25:119–34.
2. Rosenfeld PJ, Brown DM, Heier JS. . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

3. Spaide RF, Laud K, Fine HF. . Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006; 26:383–90.

4. Brown DM, Kaiser PK, Michels M. . Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.

5. Regillo CD, Brown DM, Abraham P. . Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008; 145:239–48.

6. Sonmez K, Sonmez PA, Ozkan SS, Atmaca LS. One-year outcomes of less frequent bevacizumab in age-related macular degeneration. Retina. 2011; 31:645–53.

7. Fung AE, Lalwani GA, Rosenfeld PJ. . An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–83.

8. Cohen SY, Dubois L, Tadayoni R. . Results of one-year's treatment with ranibizumab for exudative age-related macular degeneration in a clinical setting. Am J Ophthalmol. 2009; 148:409–13.

9. Lalwani GA, Rosenfeld PJ, Fung AE. . A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009; 148:43–58.e1.

10. Lew YJ, Park HJ, Lee TG. . Primary combined photodynamic therapy and intravitreal bevacizumab injection for neovascular age-related macular degeneration. J Korean Ophthalmol Soc. 2010; 51:35–41.

11. Rudnisky C, Liu C, Ng M. . Intravitreal bevacizumab alone versus combined verteporfin photodynamic therapy and intra- vitreal bevacizumab for choroidal neovascularization in age-related macular degeneration: visual acuity after 1 year of follow-up. Retina. 2010; 30:548–54.
12. Bashshur ZF, Schakal AR, El-Mollayess GM. . Ranibizumab monotherapy versus single-session verteporfin photodynamic therapy combined with as-needed ranibizumab treatment for the management of neovascular age-related macular degeneration. Retina. 2011; 31:636–44.

13. Larsen M, Schmidt-Erfùrth U, Lanzetta P. . Verteporfin plus ra- nibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month MONT BLANC study results. Ophthalmology. 2012; 119:992–1000.
14. Kaiser PK, Boyer DS, Cruess AF. . Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month results of the DENALI study. Ophthalmology. 2012; 119:1001–10.
15. Krebs I, Vécsei Marlovits V, Bodenstorfer J. . Comparison of Ranibizumab monotherapy versus combination of Ranibizumab with photodynamic therapy with neovascular age-related macular degeneration. Acta Ophthalmol. 2013; 91:e178–83.

16. Cohen SY, Creuzot-Garcher C, Darmon J. . Types of choroidal neovascularisation in newly diagnosed exudative age-related macular degeneration. Br J Ophthalmol. 2007; 91:1173–6.

17. Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neo- vascularization–verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001; 131:541–60.
18. Soubrane G, Harding SP, Wolf S, Weichselberger A. Verteporfin therapy in occult with no classic CNV due to AMD: results of the Photodynamic Therapy in Occult-Only Lesions study. Eye (Lond). 2009; 23:791–800.

19. Costagliola C, Romano M, Corte MD. . Intravitreal bevacizumab for treatment-naive patients with subfoveal occult choroidal neovascularization secondary to age-related macular degeneration: a 12-month follow-up study. Retina. 2009; 29:1227–34.
20. Furino C, Boscia F, Recchimurzo N. . Intravitreal bevacizumab for treatment-naïve subfoveal occult choroidal neovascularization in age-related macular degeneration. Acta Ophthalmol. 2009; 87:404–7.

21. Japanese Age-Related Macular Degeneration Tial (JAT) Study Group. Japanese age-related macular degeneration trial: 1-year results of photodynamic therapy with verteporfin in Japanese patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Am J Ophthalmol. 2003; 136:1049–61.
22. Costagliola C, Romano MR, Rinaldi M. . Low fluence rate photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Br J Ophthalmol. 2010; 94:180–4.

23. Sacu S, Varga A, Michels S. . Reduced fluence versus standard photodynamic therapy in combination with intravitreal triamcinolone: short-term results of a randomised study. Br J Ophthalmol. 2008; 92:1347–51.

24. Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007; 143:679–80.

25. Ciulla TA, Rosenfeld PJ. Antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2009; 20:158–65.

26. Miller JW, Schmidt-Erforth U, Sickenberg M. . Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of a single treatment in a phase 1 and 2 study. Arch Ophthalmol. 1999; 117:1161–73.
27. Kiss CG, Simader C, Michels S, Schmidt-Erfurth U. Combination of verteporfin photodynamic therapy and ranibizumab: effects on retinal anatomy, choroidal perfusion and visual function in the protect study. Br J Ophthalmol. 2008; 92:1620–7.

28. Antoszyk AN, Tuomi L, Chung CY, Singh A. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol. 2008; 145:862–74.

29. Parodi MB, Da Pozzo S, Ravalico G. Retinal pigment epithelium changes after photodynamic therapy for choroidal neovascularization in pathological myopia. Acta Ophthalmol Scand. 2007; 85:50–4.
30. Rouvas AA, Papakostas TD, Ladas ID, Vergados I. Enlargement of the hypofluorescent post photodynamic therapy treatment spot after a combination of photodynamic therapy with an intravitreal injection of bevacizumab for retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. 2008; 246:315–8.

31. Michels S, Hansmann F, Geitzenauer W, Schmidt-Erforth U. Influence of treatment parameters on selectivity of verteporfin therapy. Invest Ophthalmol Vis Sci. 2006; 47:371–6.

32. Moon SW, Kim MS, Kim ES. . Photodynamic therapy combined with intravitreal injection of vascular endothelial growth factor antibody for polypoidal choroidal vasculopathy. Ophthalmologica. 2011; 225:169–75.
Figure 1.
Changes in best-corrected visual acuity (BCVA) during the 12-month follow-up period. Patients underwent in- travitreal bevacizumab monotherapy (IVB group) or combined therapy with half-fluence rate verteporfin photodynamic therapy (PDT + IVB group) for occult choroidal neovascularization in age-related macular degeneration. Error bars represent + 1 standard error of the mean.
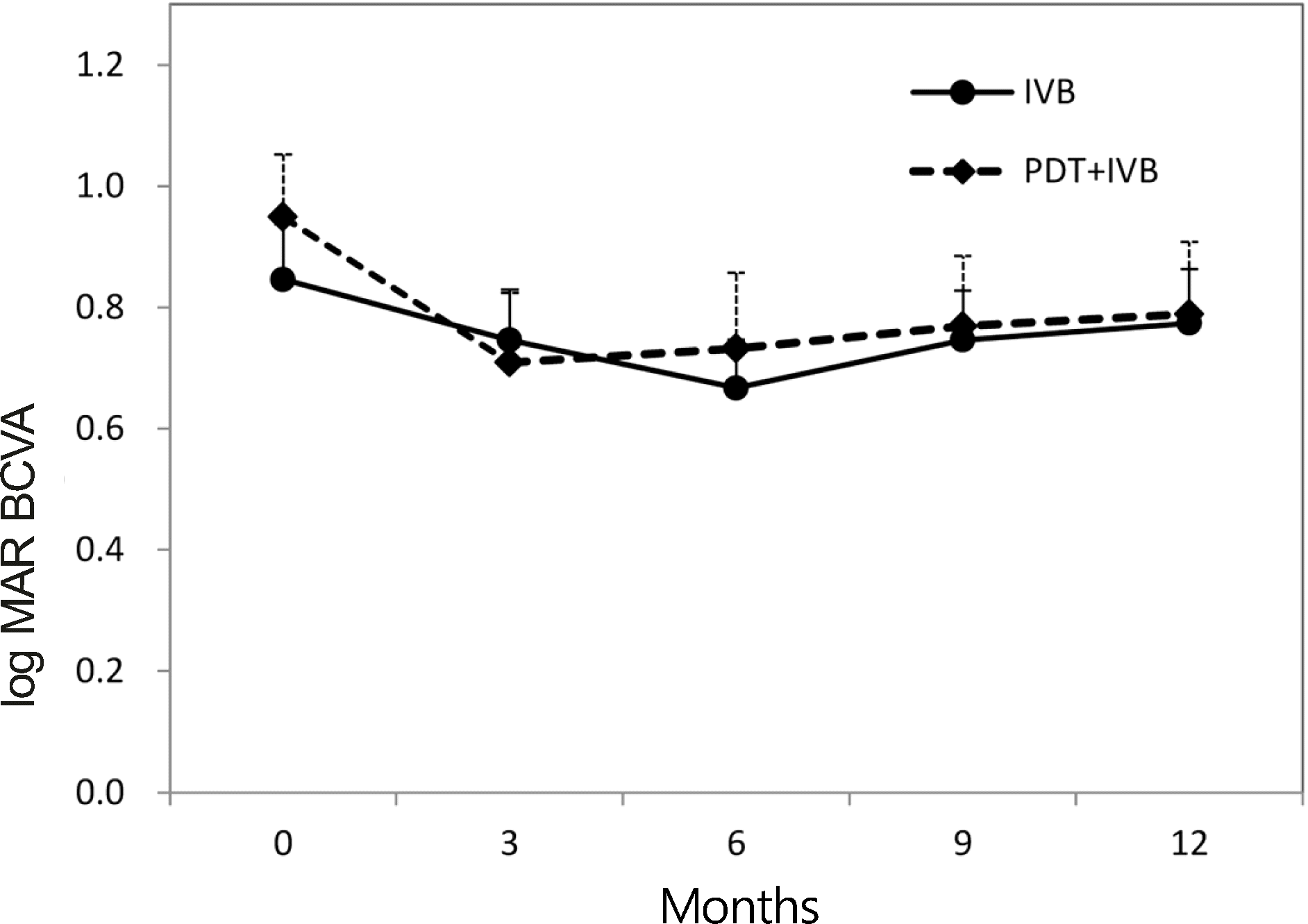
Figure 2.
Changes in central macular thickness during the 12-month follow-up period. Patients underwent intravitreal bevacizumab monotherapy (IVB group) or combined therapy with half-fluence rate verteporfin photodynamic therapy (PDT+IVB group) for occult choroidal neovascularization in age-related macular degeneration. Error bars represent +1 standard error of the mean.
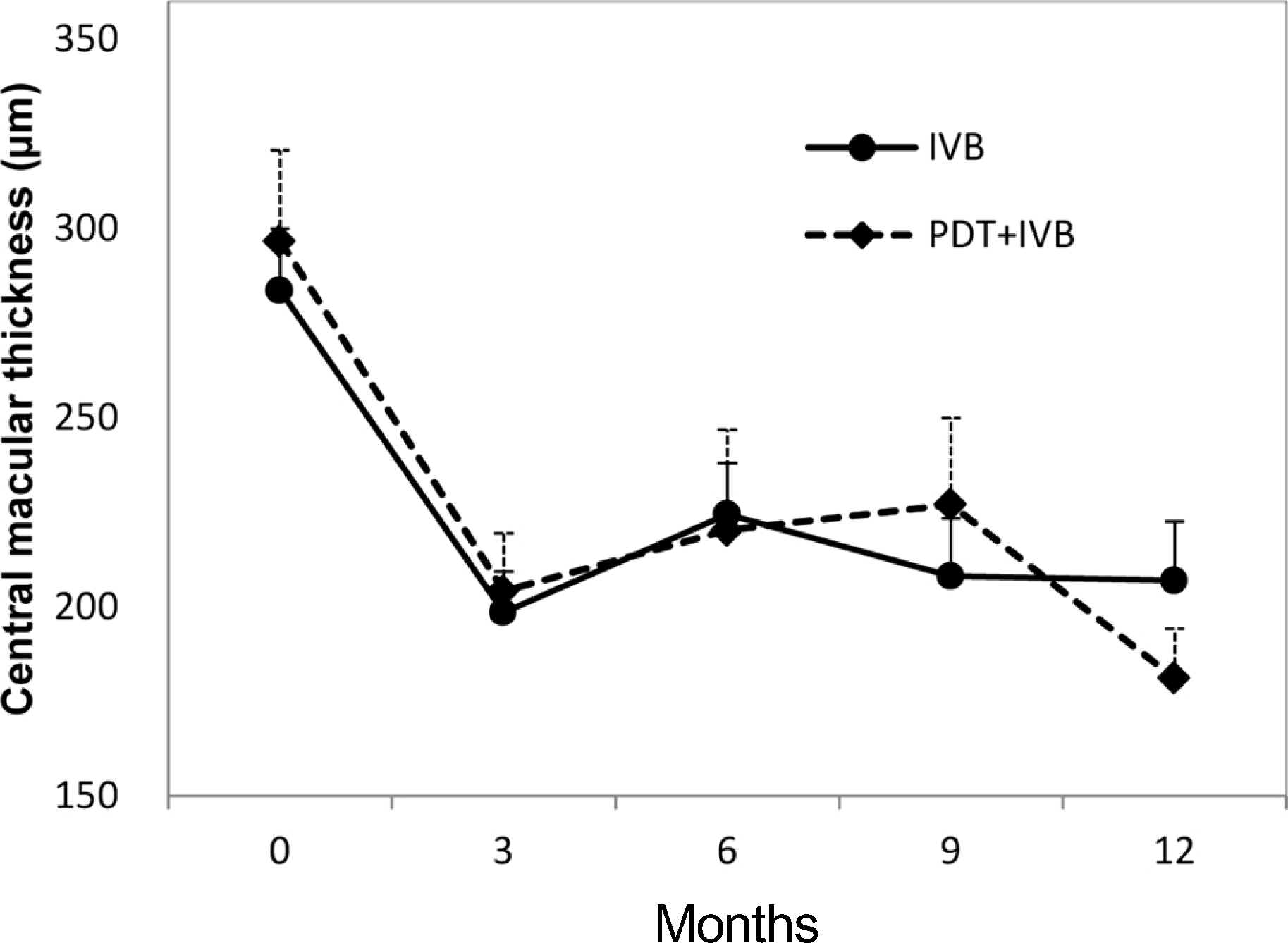
Table 1.
Baseline characteristics of the patients
| IVB group* (n = 39) | PDT+IVB group†(n = 25) | p-value | |
|---|---|---|---|
| Eyes | 39 | 25 | |
| Mean age (years) | 67.79 ± 8.18 | 71.45 ± 7.73 | 0.077 |
| No. of males/females | 24/15 | 14/11 | 0.660 |
| Diabetes (%) | 17.9 | 12.0 | 0.728‡ |
| Hypertension (%) | 38.5 | 28.0 | 0.390 |
| Subfoveal PED (%) | 46.2 | 60.0 | 0.280 |
| Subfoveal hemorrhage (%) | 46.2 | 36.0 | 0.422 |
| Greatest diameter of lesion (mm) | 3.22 ± 1.65 | 3.06 ± 1.73 | 0.716 |
| Mean BCVA (log MAR) | 0.85 ± 0.57 | 0.95 ± 0.52 | 0.457 |
| Mean CMT (μm) | 283.6 ± 101.8 | 296.6 ± 120.3 | 0.655 |
Table 2.
Changes in best-corrected visual acuity during the 12-month follow-up period
| n (eyes) | IVB group* | n (eyes) | PDT+IVB group† | p-value | |
|---|---|---|---|---|---|
| Baseline (log MAR) | 39 | 0.85 ± 0.57 | 25 | 0.95 ± 0.52 | 0.457 |
| 3 months (log MAR) | 38 | 0.75 ± 0.51 | 22 | 0.71 ± 0.54 | 0.794 |
| 6 months (log MAR) | 34 | 0.67 ± 0.47 | 21 | 0.73 ± 0.57 | 0.665 |
| 9 months (log MAR) | 36 | 0.75 ± 0.49 | 17 | 0.77 ± 0.48 | 0.870 |
| 12 months (log MAR) | 39 | 0.77 ± 0.56 | 25 | 0.79 ± 0.60 | 0.918 |
Table 3.
Results after 12 months of follow-up
| IVB group* (39 eyes) | PDT+IVB group† (25 eyes) | p-value | |
|---|---|---|---|
| Mean difference in BCVA (log MAR) from baseline | −0.07 ± 0.36 (p = 0.213) | −0.16 ± 0.38 (p = 0.046) | |
| Mean difference in CMT (μm) from baseline | −73.1 ± 102.9 (p< 0.001) | −115.4 ± 115.7 (p< 0.001) | 0.195 |
| Retreatment (%) | 74.4 | 28.0 | < 0.001 |
| Mean number of additional IVB injections | 1.59 ± 1.33 | 0.52 ± 1.01 | 0.001 |
Table 4.
Changes in central macular thickness during the 12-month follow-up period
| n (eyes) | IVB group* | n (eyes) | PDT+IVB group† | p-value | |
|---|---|---|---|---|---|
| Baseline (μm) | 39 | 283.6 ± 101.8 | 25 | 296.6 ± 120.3 | 0.655 |
| 3 months (μm) | 38 | 198.5 ± 66.3 | 22 | 204.1 ± 70.0 | 0.764 |
| 6 months (μm) | 34 | 224.4 ± 75.2 | 21 | 220.2 ± 110.1 | 0.888 |
| 9 months (μm) | 36 | 208.1 ± 85.9 | 17 | 227.0 ± 82.7 | 0.499 |
| 12 months (μm) | 39 | 206.9 ± 89.3 | 25 | 181.1 ± 56.8 | 0.209 |
Table 5.
Visual changes from baseline during the 12-month follow-up period
|
IVB group *(39 eyes) |
PDT+IVB group† (25 eyes) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Visual Changes | 3 months (n = 38) (%) | 6 months (n = 34) (%) | 9 months (n = 36) (%) | 12 months (n = 39) (%) | 3 months (n = 22) (%) | 6 months (n = 21) (%) | 9 months (n = 17) (%) | 12 months (n = 25) (%) |
| Gain of ≥ 0.3 log MAR | 11 (28.9) | 10 (29.4) | 8 (22.2) | 9 (23.1) | 8 (36.4) | 8 (38.1) | 8 (47.1) | 9 (23.1) |
| No change or gain of < 0.3 log MAR | 19 (50.0) | 15 (44.1) | 19 (52.8) | 17 (43.6) | 10 (45.5) | 9 (42.9) | 4 (23.5) | 17 (43.6) |
| Loss of < 0.3 log MAR | 5 (13.2) | 5 (14.7) | 7 (19.4) | 10 (25.6) | 2 (9.1) | 2 (9.5) | 2 (11.8) | 10 (25.6) |
| Loss of ≥ 0.3 log MAR | 3 (7.9) | 4 (11.8) | 2 (5.6) | 3 (7.7) | 2 (9.1) | 2 (9.5) | 3 (17.6) | 3 (7.7) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download