Abstract
Purpose
To evaluate the efficacy of topical 0.1% sodium hyaluronate (HA) and 0.05% cyclosporine A (CsA) on tear film parameters after cataract surgery.
Methods
A total of 109 eyes of 86 patients who underwent cataract surgery were divided into four groups: 74 eyes without dry eye syndrome (groups 1 and 2) and 35 eyes with dry eye syndrome (groups 3 and 4). Groups 1 (37 eyes) and 3 (17 eyes) used 0.1% HA only, while Groups 2 (37 eyes) and 4 (18 eyes) used both 0.1% HA and 0.05% CsA. Tear film breakup time (BUT), Schirmer's test, tear clearance rate (TCR) and keratoepitheliopathy were evaluated before surgery and 1 week, 1 month, 2, 3 and 6 months after surgery.
References
1. The definition of classification of dry eye diseases: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92.
2. Horwath-Winter J, Vidic B, Schwantzer G, Schmut O. Early changes in corneal sensation, ocular surface integrity, and tear-film function after laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2004; 30:2316–21.

3. Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007; 26(9 Suppl 1):S16–20.

4. Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000; 19:644–9.
5. Nussenblatt RB, Palestine AG. Cyclosporine: immunology, pharmacology and therapeutic uses. Surv Ophthalmol. 1986; 31:159–69.

6. Hemady R, Tauber J, Foster CS. Immunosuppressive drugs in immune and inflammatory ocular disease. Surv Ophthalmol. 1991; 35:369–85.

7. Jabs DA, Wingard J, Green WR, et al. The eye in bone marrow transplantation. III. Conjunctival graft-vs-host disease. Arch Ophthalmol. 1989; 107:1343–8.
8. Bhan AK, Fujikawa LS, Foster CS. T-cell subsets and Langerhans cells in normal and diseased conjunctiva. Am J Ophthalmol. 1982; 94:205–12.

9. Gilbard JP, Rossi SR, Azar DT, Heyda KG. Effect of punctal occlusion by Freeman silicone plug insertion on tear osmolarity in dry eye disorders. CLAO J. 1989; 15:216–8.
10. Lee EH, Jang JW, Lew HM. The changes of tear osmolarity and protein after silicone punctal plug insertion in dry eye. J Korean Ophthalmol Soc. 2001; 42:1509–14.
11. Yen MT, Pflugfelder SC, Feuer WJ. The effect of punctal occlusion on tear production, tear clearance, and ocular surface sensation in normal subjects. Am J Ophthalmol. 2001; 131:314–23.

12. Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008; 27:64–9.
13. Wilson SE, Perry HD. Long-term resolution of chronic dry eye symptoms and signs after topical cyclosporine treatment. Ophthalmology. 2007; 114:76–9.

14. Roberts CW, Carniglia PE, Brazzo BG. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea. 2007; 26:805–9.

15. Raphael M, Bellefqih S, Piette JC, et al. Conjunctival biopsy in Sjögren's syndrome: correlations between histological and immunohistochemical features. Histopathology. 1988; 13:191–202.

16. Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000; 118:1489–96.
17. O'Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004; 4:314–9.
18. Small DS, Acheampong A, Reis B, et al. Blood concentrations of cyclosporin a during long-term treatment with cyclosporin a ophthalmic emulsions in patients with moderate to severe dry eye disease. J Ocul Pharmacol Ther. 2002; 18:411–8.

19. Laibovitz RA, Solch S, Andriano K, et al. Pilot trial of cyclosporine 1% ophthalmic ointment in the treatment of keratoconjunctivitis sicca. Cornea. 1993; 12:315–23.

20. Turner K, Pflugfelder SC, Ji Z, et al. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000; 19:492–6.

21. Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000; 107:967–74.
22. Moon JW, Lee HJ, Shin KC, et al. Short term effects of topical cyclosporine and viscoelastic on the ocular surfaces in patients with dry eye. Korean J Ophthalmol. 2007; 21:189–94.

23. Xu KP, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea. 1996; 15:235–9.

24. Hiroko BM. Cataract surgery in the presence of other ocular comorbidities. Steinert RF, editor. Cataract Surgery: Technique, Complication and Management. 2nd ed.Philadelphia: Saunders;2004. chap. 32.
25. Walker TD. Benzalkonium toxicity. Clin Experiment Ophthalmol. 2004; 32:657.
Figure 1.
Changes in tear film breakup time, Schirmer's test, tear clearance rate and keratoepitheliopathy after cataract surgery in the non-dry eye groups. HA = hyaluronate; CsA = cyclosporine A.* p < 0.05 compared with each group.
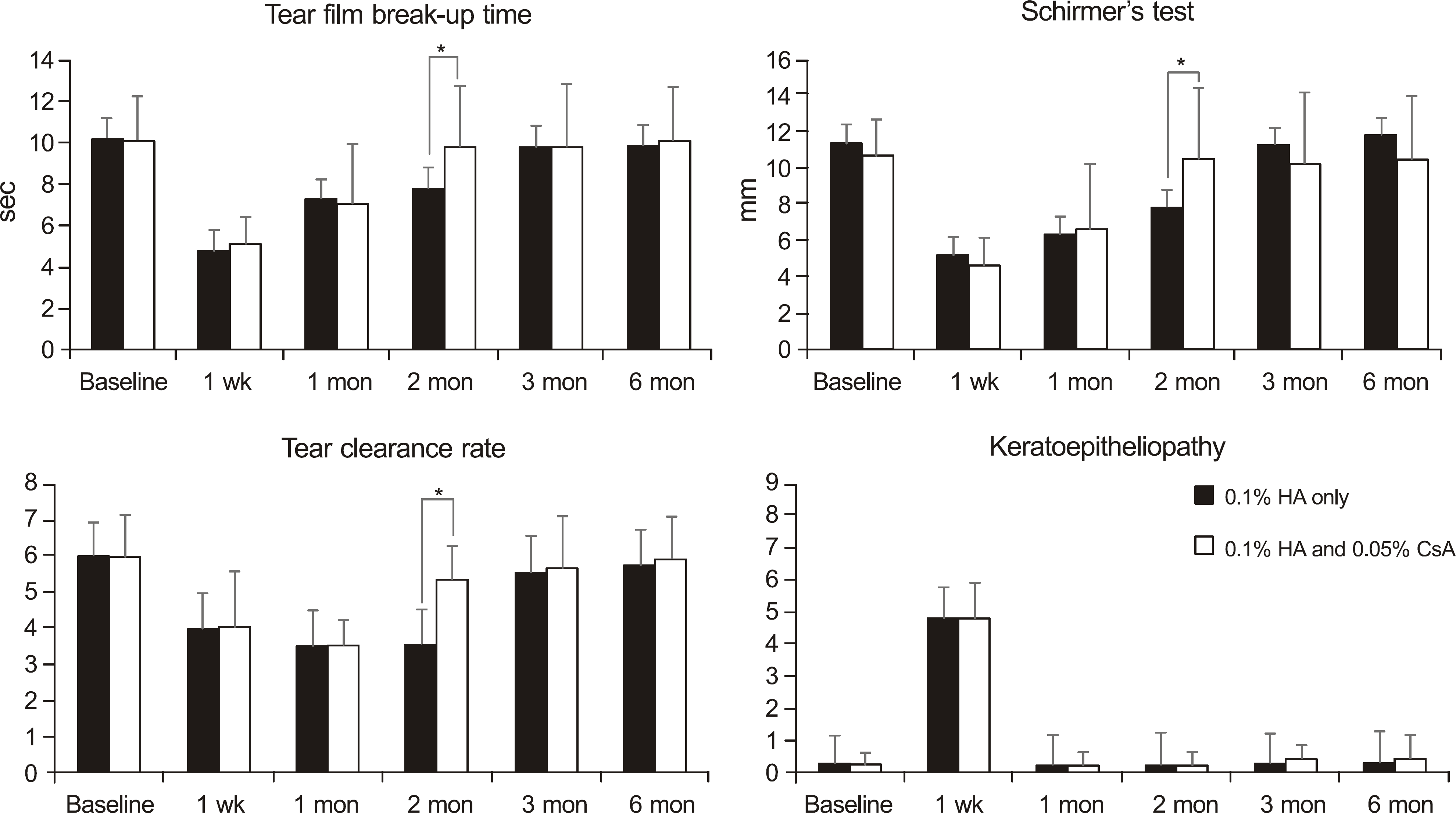
Figure 2.
Changes in tear film breakup time, Schirmer's test, tear clearance rate and keratoepitheliopathy after cataract surgery in the dry eye groups. HA = hyaluronate; CsA = cyclosporine A.* p < 0.05 compared with each group.
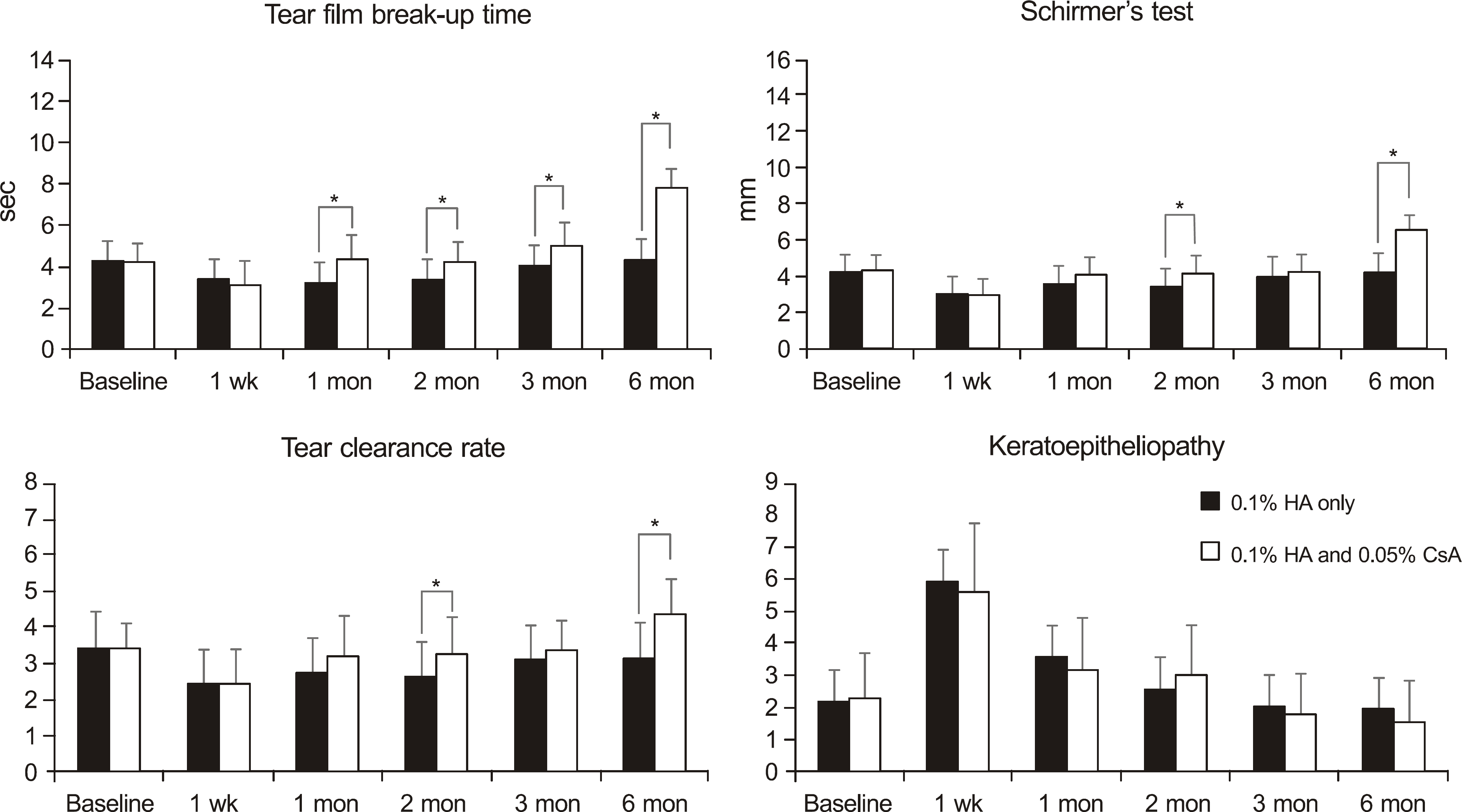
Table 1.
Changes in the tear film and ocular surface parameters after cataract operation in patients without dry eye
| Baseline | 1 week after treatment | 1 month after treatment | 2 months after treatment | 3 months after treatment | 6 months after treatment | ||
|---|---|---|---|---|---|---|---|
| Group 1 | Tear film breakup time (sec) | 10.19 ± 2.17 | 4.78 ± 1.27* | 7.24 ± 2.89* | 7.81 ± 2.93* | 9.81 ± 3.04 | 9.89 ± 2.58 |
| Schirmer's test (mm) | 11.41 ± 1.98 | 5.24 ± 1.55* | 6.38 ± 3.52* | 7.86 ± 3.92* | 11.30 ± 3.97 | 11.86 ± 3.58 | |
| Tear clearance rate ((Log2)-1) | 5.95 ± 1.18 | 3.97 ± 1.52* | 3.49 ± 0.73* | 3.54 ± 0.93* | 5.54 ± 1.45 | 5.73 ± 1.22 | |
| Keratoepitheliopathy score | 0.16 ± 0.37 | 4.78 ± 1.11* | 0.24 ± 0.43 | 0.24 ± 0.43 | 0.24 ± 0.43 | 0.27 ± 0.73 | |
| Group 2 | Tear film breakup time (sec) | 10.08 ± 1.55 | 5.14 ± 1.49* | 7.05 ± 2.26* | 9.86 ± 2.53 | 9.86 ± 2.69 | 10.14 ± 2.04 |
| Schirmer's test (mm) | 10.76 ± 2.60 | 4.68 ± 1.83* | 6.70 ± 3.19* | 10.54 ± 3.07 | 10.32 ± 2.65 | 10.51 ± 2.55 | |
| Tear clearance rate ((Log2)-1) | 5.97 ± 1.14 | 4.05 ± 1.45* | 3.51 ± 0.69* | 5.35 ± 1.42 | 5.65 ± 1.25 | 5.89 ± 1.43 | |
| Keratoepitheliopathy score | 0.24 ± 0.43 | 4.81 ± 1.73* | 0.19 ± 0.40 | 0.24 ± 0.43 | 0.41 ± 0.86 | 0.43 ± 0.87 |
Table 2.
Changes in the tear film and ocular surface parameters after cataract operation in patients with dry eye
| Baseline | 1 week after treatment | 1 month after treatment | 2 months after treatment | 3 months after treatment | 6 months after treatment | ||
|---|---|---|---|---|---|---|---|
| Group 3 | Tear film breakup time (sec) | 4.16 ± 0.83 | 3.26 ± 1.15* | 3.11 ± 1.20* | 3.26 ± 0.99* | 3.95 ± 1.08 | 4.21 ± 0.92 |
| Schirmer's test (mm) | 4.11 ± 0.88 | 2.89 ± 0.94* | 3.47 ± 0.90* | 3.37 ± 0.96* | 3.95 ± 0.97 | 4.21 ± 0.85 | |
| Tear clearance rate ((Log2)-1) | 3.47 ± 0.70 | 2.42 ± 0.96* | 2.74 ± 1.10* | 2.63 ± 1.01* | 3.11 ± 0.81 | 3.16 ± 0.96 | |
| Keratoepitheliopathy score | 2.21 ± 1.36 | 5.95 ± 2.15* | 3.58 ± 1.61* | 2.58 ± 1.54 | 2.05 ± 1.31 | 1.95 ± 1.31 | |
| Group 4 | Tear film breakup time (sec) | 4.15 ± 0.88 | 3.05 ± 1.10* | 4.25 ± 0.79 | 4.15 ± 0.75 | 4.95 ± 1.73 | 7.75 ± 1.52† |
| Schirmer's test (mm) | 4.20 ± 0.89 | 2.85 ± 0.81* | 4.05 ± 0.83 | 4.10 ± 0.85 | 4.15 ± 0.88 | 6.50 ± 2.01† | |
| Tear clearance rate ((Log2)-1) | 3.45 ± 0.69 | 2.45 ± 0.89* | 3.25 ± 0.79 | 3.30 ± 0.66 | 3.40 ± 0.68 | 4.40 ± 1.93 | |
| Keratoepitheliopathy score | 2.35 ± 1.31 | 5.60 ± 1.79* | 3.20 ± 1.82 | 3.05 ± 1.54 | 1.80 ± 1.28 | 1.55 ± 1.15 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download