Abstract
Purpose
To investigate the effects of mitomycin C on the scleral collagen surfaces using atomic force microscopy (AFM).
Methods
Two non-contact mode AFM machines were used to observe changes in the morphological characteristics of human scleral surfaces before and after one, three, and five minutes of 0.02% mitomycin C application. Based on AFM topography and deflection images of the collagen fibril, the morphological characteristics of scleral fibrils including the fibril diameter and D-period were measured using the line profile.
Results
The sclera collagen fibril treated with 0.02% mitomycin C for one minute did not show any significant increases in mean fibril diameter (155.04 ± 17.46 nm) or mean D-periodicity (70.02 ± 3.33 nm), compared to those of the control group. However, the scleral collagen fibrils treated with 0.02% mitomycin C for three and five minutes showed significant increases in mean fibril diameter (182.33 ± 16.33 nm, 199.20 ± 12.40 nm, respectively) and mean D-periodicity (70.27 ± 13.66 nm, 72.75 ± 19.32 nm, respectively), compared to those of the control group.
Conclusions
The present study examined the structural changes in the scleral collagen fibrils before and after mitomycin C application according to atomic force microscopy. The results indirectly suggest that three or more minutes of 0.02% mitomycin C application affects the morphology of scleral collagen.
References
1. Danshiitsoodol N, de Pinho CA, Matoba Y, et al. The mitomycin C (MMC)-binding protein from MMC-producing microorganisms protects from the lethal effect of bleomycin: crystallographic analysis to elucidate the binding mode of the antibiotic to the protein. J Mol Biol. 2006; 360:398–408.

2. Mao Y, Varoglu M, Sherman DH. Molecular characterization and analysis of the biosynthetic gene cluster for the antitumor antibiotic mitomycin C from Streptomyces lavendulae NRRL 2564. Chem Biol. 1999; 6:251–63.

3. Oum BS, Lee JS. The effect of Mitomycin C (MMC) on inhibition of cellular proliferation and type_I collagen, laminin synthesis of pterygial mesenchymal cell. J Korean Ophthalmol Soc. 1999; 40:712–20.
4. Willems EW, Nooter K, Verweij J. Antitumor antibiotics. Chabner BA, Longo DL, editors. Cancer Chemotherapy & Biotherapy: Principles and Practice. 4th ed.Philadelphia: Lippincott Williams & Wilkins;2006. 1:chap. 16.
5. Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-in-fusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002; 20:1996–2004.

6. Lee TS, Rhee K. The effect of Mitomycin C eyedrops on prevention of intermal ostium obstruction after endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 1998; 39:1915–20.
7. Park DJ, Kwak MS. The effect of Mitomycin C on the success rate of endoscopic dacryo cystorhinostomy. J Korean Ophthalmol Soc. 2000; 41:1674–9.
8. Anderson RL, Edwards JJ. Indications, complications and results with silicone stents. Ophthalmology. 1979; 86:1474–87.

9. Tarr KH, Constable IJ. Late complications of pterygium treatment. Br J Ophthalmol. 1980; 64:496–505.

10. Rubinfeld RS, Pfister RR, Stein RM, et al. Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology. 1992; 99:1647–54.

11. Wan Norliza WM, Raihan IS, Azwa JA, Ibrahim M. Scleral melting 16 years after pterygium excision with topical Mitomycin C adjuvant therapy. Cont Lens Anterior Eye. 2006; 29:165–7.

12. Song HY, Im JS, Kwak JY. Acellular dermal allograft transplantation in patients with scleromalacia after pterygium excision. J Korean Ophthalmol Soc. 2008; 49:1685–9.

13. Na YS, Joo MJ, Kim JH. Results of scleral allografting on scleral necrosis following pterygium excision. J Korean Ophthalmol Soc. 2005; 46:402–9.
14. Anduze AL, Burnett JM. Indications for and complications of mi-tomycin-C in pterygium surgery. Ophthalmic Surg Lasers. 1996; 27:667–73.

15. Keeley FW, Morin JD, Vesely S. Characterization of collagen from normal human sclera. Exp Eye Res. 1984; 39:533–42.

16. Spitznas M, Luciano L, Reale E. Fine structure of rabbit scleral collagen. Am J Ophthalmol. 1970; 69:414–8.

18. Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991; 32:2244–58.
19. Marshall GE, Konstas AG, Lee WR. Collagens in the aged human macular sclera. Curr Eye Res. 1993; 12:143–53.

20. Lin Z, Chen X, Ge J, et al. Effects of direct intravitreal dopamine injection on sclera and retina in form-deprived myopic rabbits. J Ocul Pharmacol Ther. 2008; 24:543–50.

21. Meek KM, Fullwood NJ. Corneal and scleral collagens–a micro-scopist's perspective. Micron. 2001; 32:261–72.

22. Fullwood NJ, Hammiche A, Pollock HM, et al. Atomic force microscopy of the cornea and sclera. Curr Eye Res. 1995; 14:529–35.

23. Meller D, Peters K, Meller K. Human cornea and sclera studied by atomic force microscopy. Cell Tissue Res. 1997; 288:111–8.

24. Yamamoto S, Hitomi J, Sawaguchi S, et al. Observation of human corneal and scleral collagen fibrils by atomic force microscopy. Jpn J Ophthalmol. 2002; 46:496–501.

25. Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996; 316:1–11.

26. Kwak JJ, Lee DH, Lew HM. Endoscopic dacryocystorhinostomy with Mitomycin-C application. J Korean Ophthalmol Soc. 1998; 39:2211–7.
27. Lee KS, Byun YJ. Dacryocystorhinostomy with intraoperative Mitomycin C. J Korean Ophthalmol Soc. 1998; 39:1909–14.
28. Kim JH, Lee HB, Yoon DK. Scleral grafts fer the cases of scleral perforation, scleral ectasia and scleral necrosis. J Korean Ophthalmol Soc. 1978; 19:55–64.
29. Manning CA, Kloess PM, Diaz MD, Yee RW. Intraoperative mitomycin in primary pterygium excision. A prospective, randomized trial. Ophthalmology. 1997; 104:844–8.
30. Khaw PT, Sherwood MB, Doyle JW, et al. Intraoperative and post operative treatment with 5-fluorouracil and mitomycin-c: long term effects in vivo on subconjunctival and scleral fibroblasts. Int Ophthalmol. 1992; 16:381–5.

31. Rubinfeld RS, Pfister RR, Stein RM, et al. Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology. 1992; 99:1647–54.

Figure 1.
(A) Atomic force microscopy (AFM) block diagram, and (B) force vs. distance and relative zone modes.
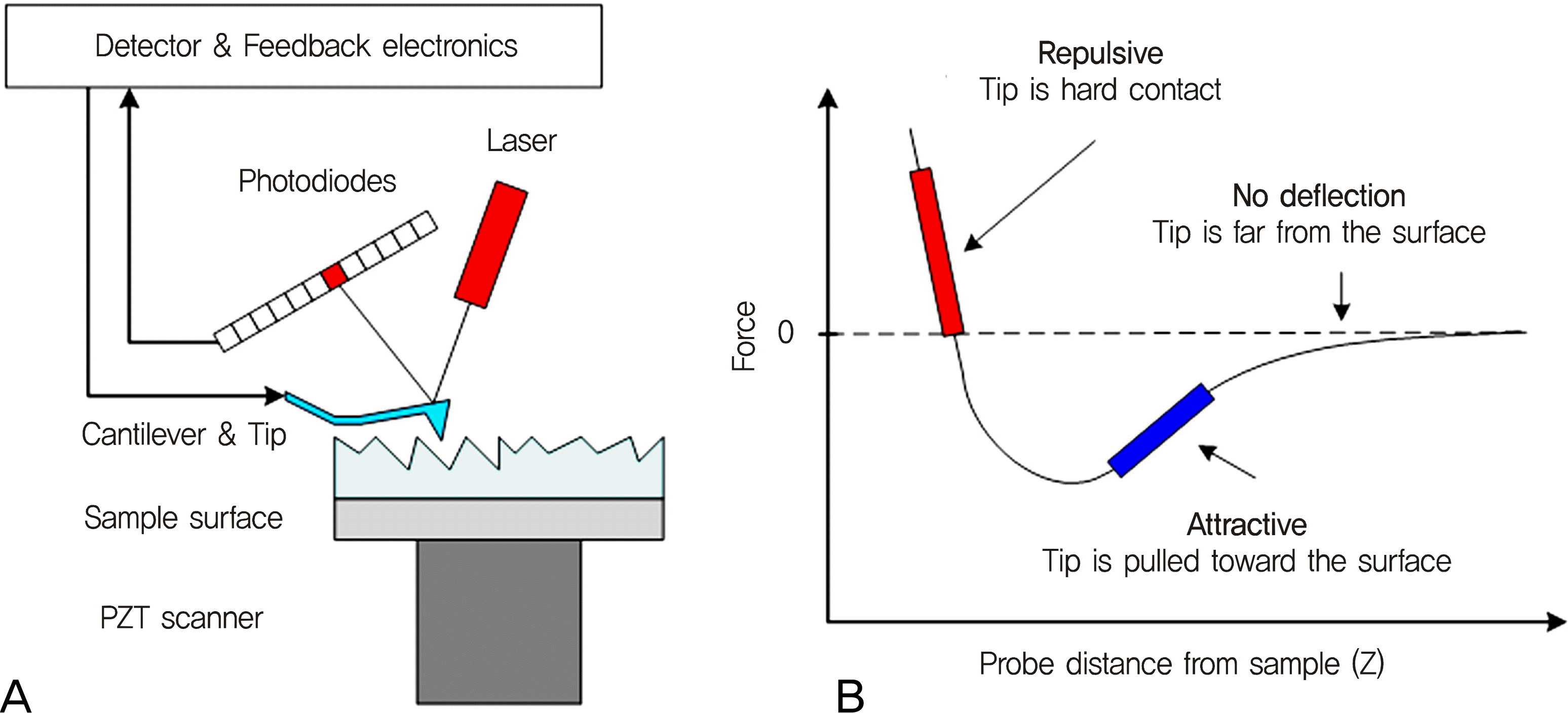
Figure 2.
Representative atomic force microscopy topography images of the dehydrated human sclera in various scan sizes such as (A) 20 × 20 µm2, (B) 5 × 5 µm2, and (C) 1 × 1 µm2.

Figure 3.
Representative three-dimensional images of the dehydrated human sclera in various scan sizes such as (A) 20 × 20 µm2, (B) 5 × 5 µm2, and (C) 1 × 1 µm2.
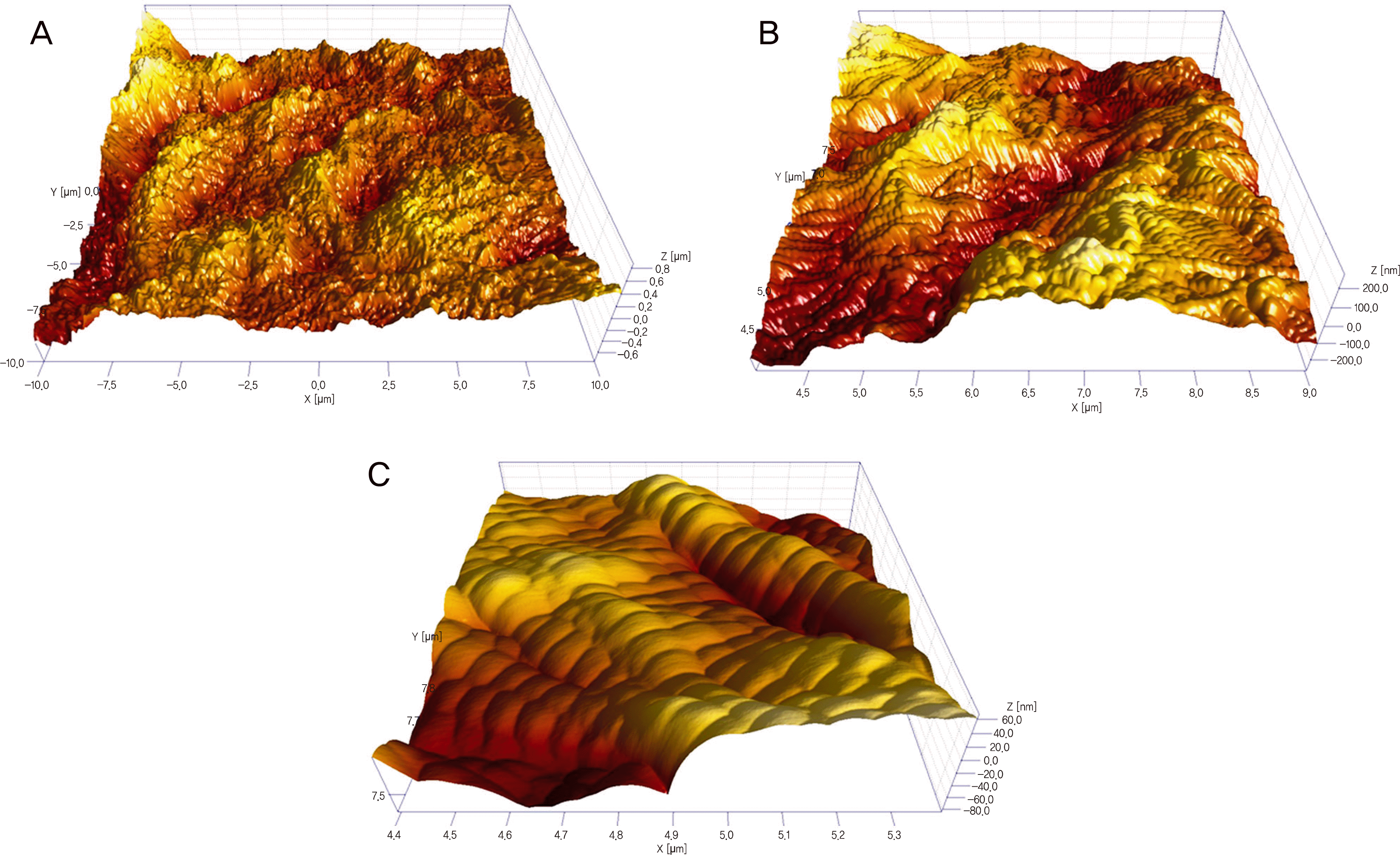
Figure 4.
Representative examples of the diameter (C) and D-banding (D) measurements using line profiling plots on AFM topography (A) and deflection images (B) of the dehydrated scleral collagen fibrils in a scan size of 5 × 5 μ m2.
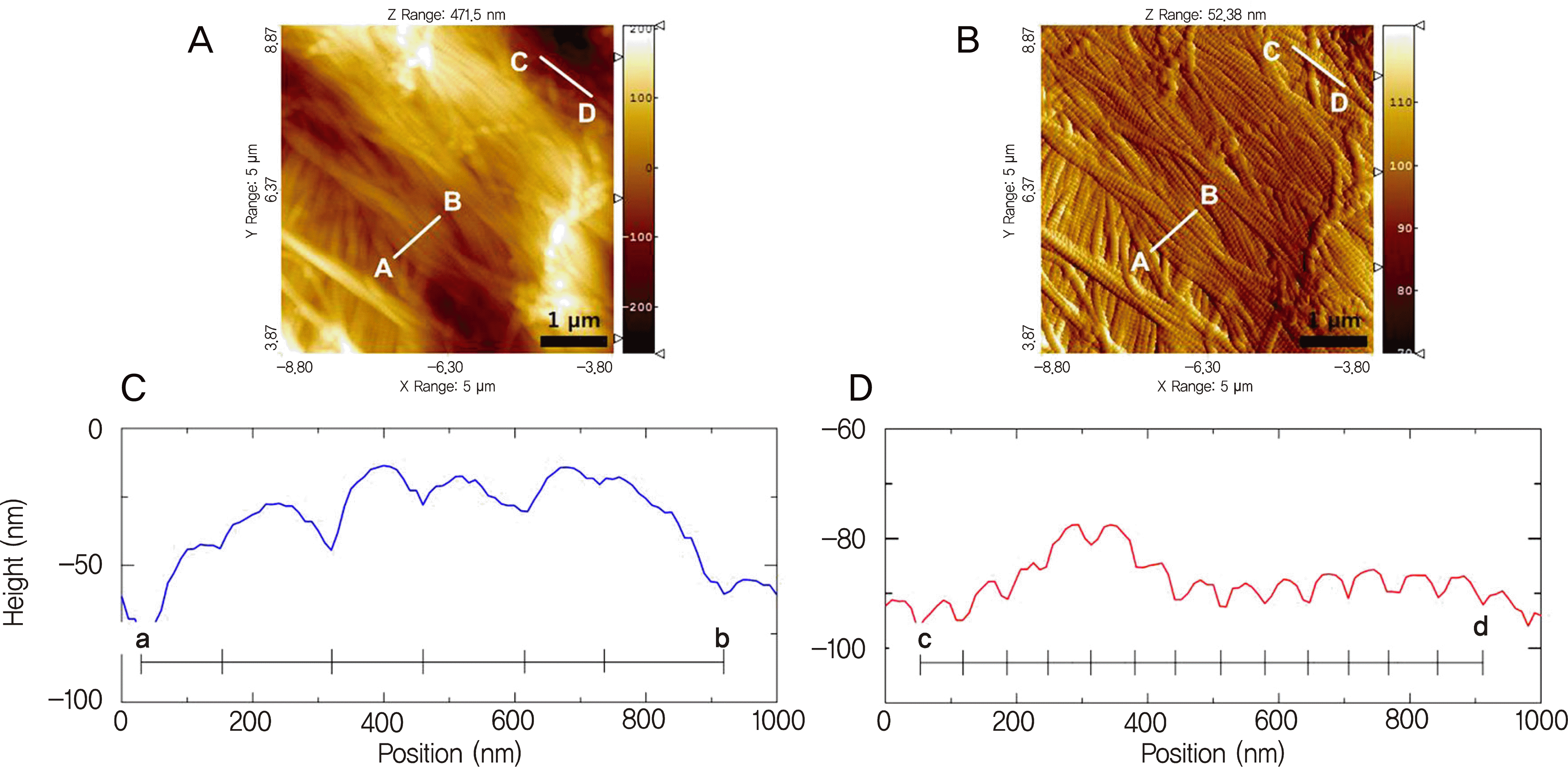
Figure 5.
Representative AFM topography (A), deflection (B), and three-dimensional images (C) of the dehydrated scleral collagen fibrils in a scan size of 5 × 5 μ m2.
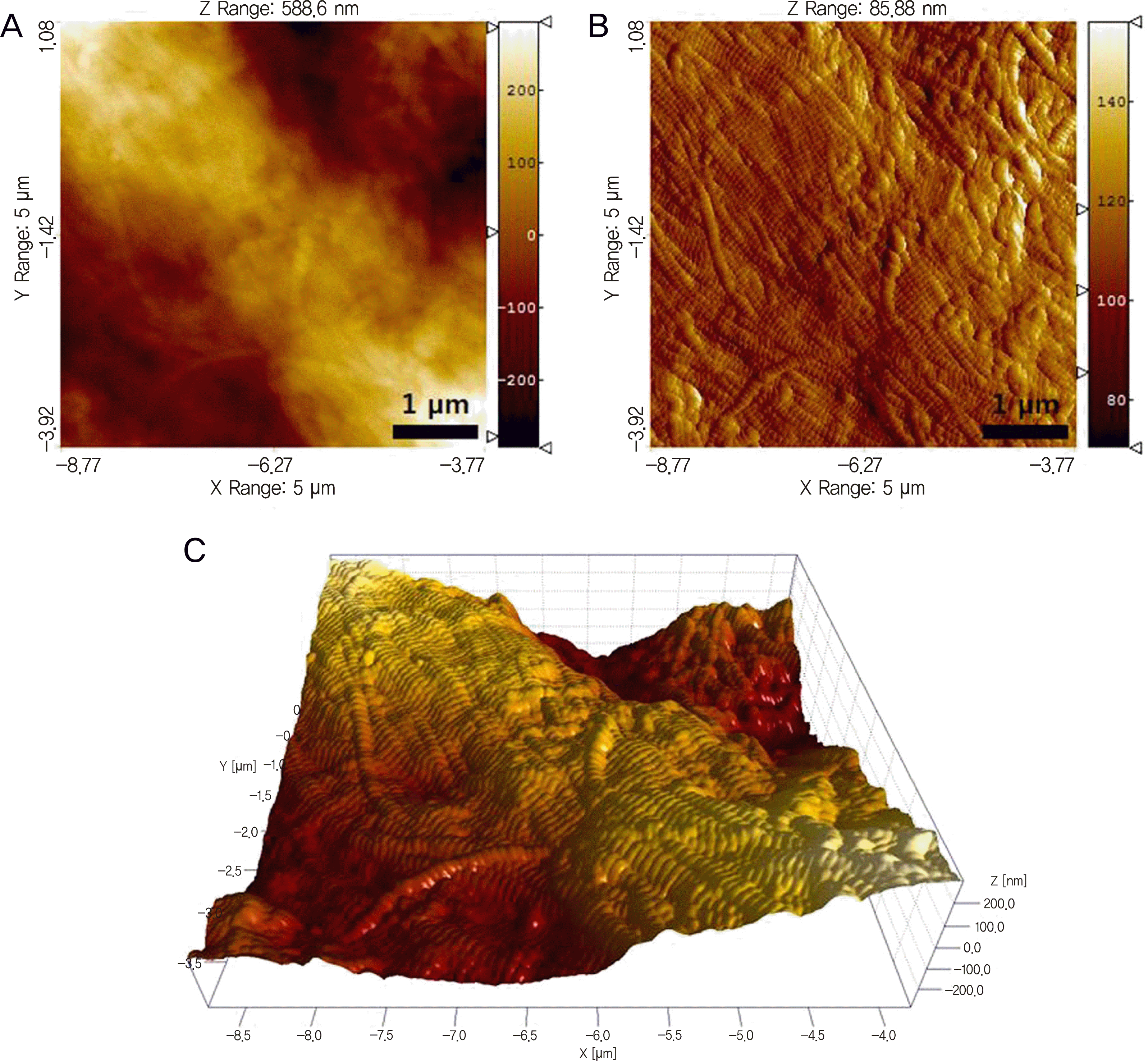
Figure 6.
Representative AFM topography (A), deflection (B), and three-dimensional images (C) of the dehydrated scleral collagen fibrils with 0.02% mitomycin C application for 5 minutes in a scan size of 5 × 5 μ m2.
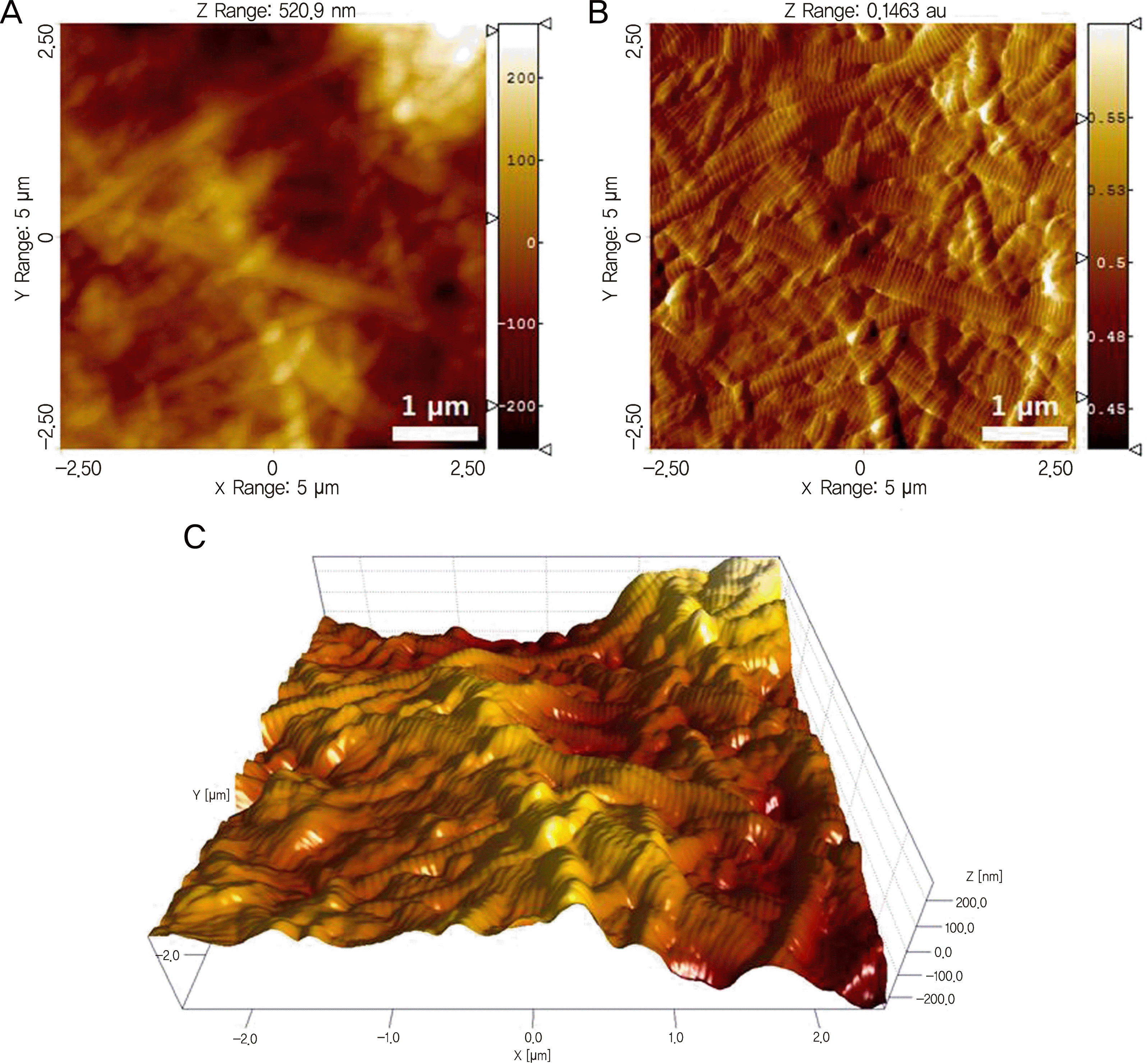
Table 1.
Morphological changes in the sclera fibrils for control and MMC-treated groups
| Parameter | Control | MMC 1 min | MMC 3 min | MMC 5 min |
|---|---|---|---|---|
| Diameter (mean ± SD, nm) | 145.22 ± 17.78 | 155.04 ± 17.46 | 182.33 ± 16.33* | 199.20 ± 12.40† |
| D-banding (mean ± SD, nm) | 69.14 ± 14.15 | 70.02 ± 3.33 | 70.27 ± 13.66 | 72.75 ± 19.32 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download