Abstract
Purpose
To report the effectiveness of an autologous tragal perichondrium graft for a necrotizing scleritis case which was refractory to conventional surgery.
Case summary
A 75-year-old woman was referred to our clinic with recurrent necrotizing scleritis of the left eye which occurred after pterygium removal five years earlier. The patient underwent scleral graft, pericardium graft, and amniotic membrane graft in other clinics; however, necrosis of the sclera progressed. The best corrected visual acuity was 0.06, and choroidal tissue was nearly exposed below the melted pericardium graft in the nasal area. The authors harvested tragal perichondrium from the right ear, and the scleral defect was successfully reconstructed with an autologous tragal perichondium graft. The graft showed rapid epithelization and neovascularization within a week and conjunctivalization after three months. No complications have been observed up to one year after surgery.
References
1. O'Donoghue E, Lightman S, Tuft S, Watson P. Surgically induced necrotising sclerokeratitis (SINS) – precipitating factors and response to treatment. Br J Ophthalmol. 1992; 76:17–21.
2. Kim JH. Scleral grafting on necrotic scleritis following pterygium excision. J Korean Ophthalmol Soc. 1982; 23:29–39.
3. Na YS, Joo MJ, Kim JH. Results of scleral allografting on scleral necrosis following pterygium excision. J Korean Ophthalmol Soc. 2005; 46:402–9.
4. Ahn BH, Lee EJ, Sung KH. The use of a temporal muscle fascia in the treatment of scleral defect. J Korean Ophthalmol Soc. 1983; 24:785–91.
5. Gwag J-Y, Jang HG. Autogenous temporalis fascia grafting and conjunctival flap transposition in scleromalacia after pterygium excision. J Korean Ophthalmol Soc. 2004; 45:180–6.
6. Lee CO, Jong SH, Lee JJ. Autologous simple conjunctival graft and conjunctiva / tenon graft on focal scleromalacia. J Korean Ophthalmol Soc. 1997; 38:1737–41.
7. Mauriello JA Jr, Fiore PM, Pokorny KS, Cinotti DJ. Use of split-thickness dermal graft in the surgical treatment of corneal and scleral defects. Am J Ophthalmol. 1988; 105:244–7.

8. Cavaliere M, Mottola G, Rondinelli M, Iemma M. Tragal cartilage in tympanoplasty: anatomic and functional results in 306 cases. Acta Otorhinolaryngol Ital. 2009; 29:27–32.
9. Yoon CH, Kim NJ, Lee MJ, et al. Correction of lower lid retraction using autologous ear cartilage graft. J Korean Ophthalmol Soc. 2011; 52:136–40.

10. Nigro MV, Friedhofer H, Natalino RJ, Ferreira MC. Comparative analysis of the influence of perichondrium on conjunctival epithelialization on conchal cartilage grafts in eyelid reconstruction: experimental study in rabbits. Plast Reconstr Surg. 2009; 123:55–63.

11. Hayasaka S, Noda S, Yamamoto Y, Setogawa T. Postoperative instillation of Mitomycin C in the treatment of recurrent pterygium. Ophthalmic Surg. 1989; 20:580–3.

12. Galanopoulos A, Snibson G, O'Day J. Necrotising anterior scleritis after pterygium surgery. Aust NZ J Ophthalmol. 1994; 22:167–73.

13. Tarr KH, Constable IJ. Late complications of pterygium treatment. Br J Ophthalmol. 1980; 64:496–505.

14. Rubinfeld RS, Pfister RR, Stein RM, et al. Serious complications of topical Mitomycin-C after pterygium surgery. Ophthalmology. 1992; 99:1647–54.

15. Aronson SB, Elliott JH. Ocular Inflammation. 1st ed.St. Louis: Mosby;1972. p. 210.
16. Jeoung JW, Yoon YM, Lee JL, et al. The effect of amniotic membrane transplantation on the treatment of necrotizing scleritis after pterygium excision. J Korean Ophthalmol Soc. 2004; 45:1981–8.
Figure 1.
A photograph of 75-year-old woman with recurrent necrotizing scleritis of the left eye which occurred after pterygium removal 5 years earlier. (A) After undergoing sclera graft, pericardium graft, and amniotic membrane graft, sclera necrosis has still progressed and choroid is bulging out through the melted graft tissue. (B) Choroid is being exposed after removing the pericardium graft during surgery. (C) Autologous tragal perichondrium graft has been made.
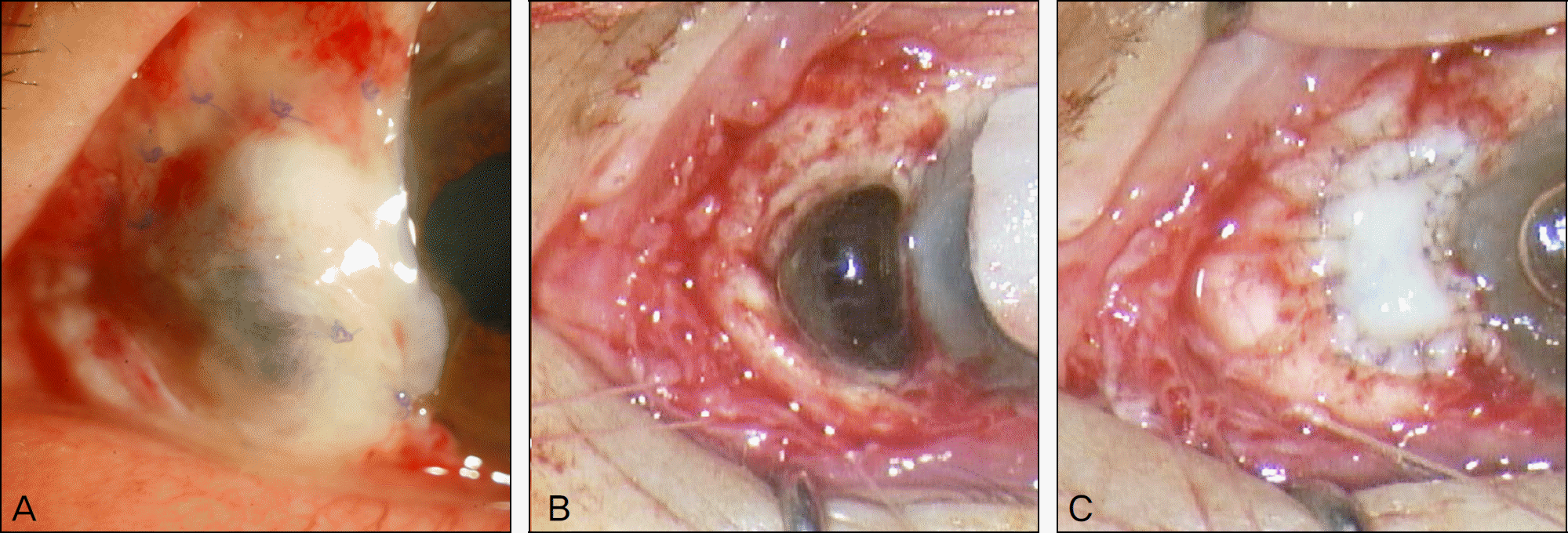
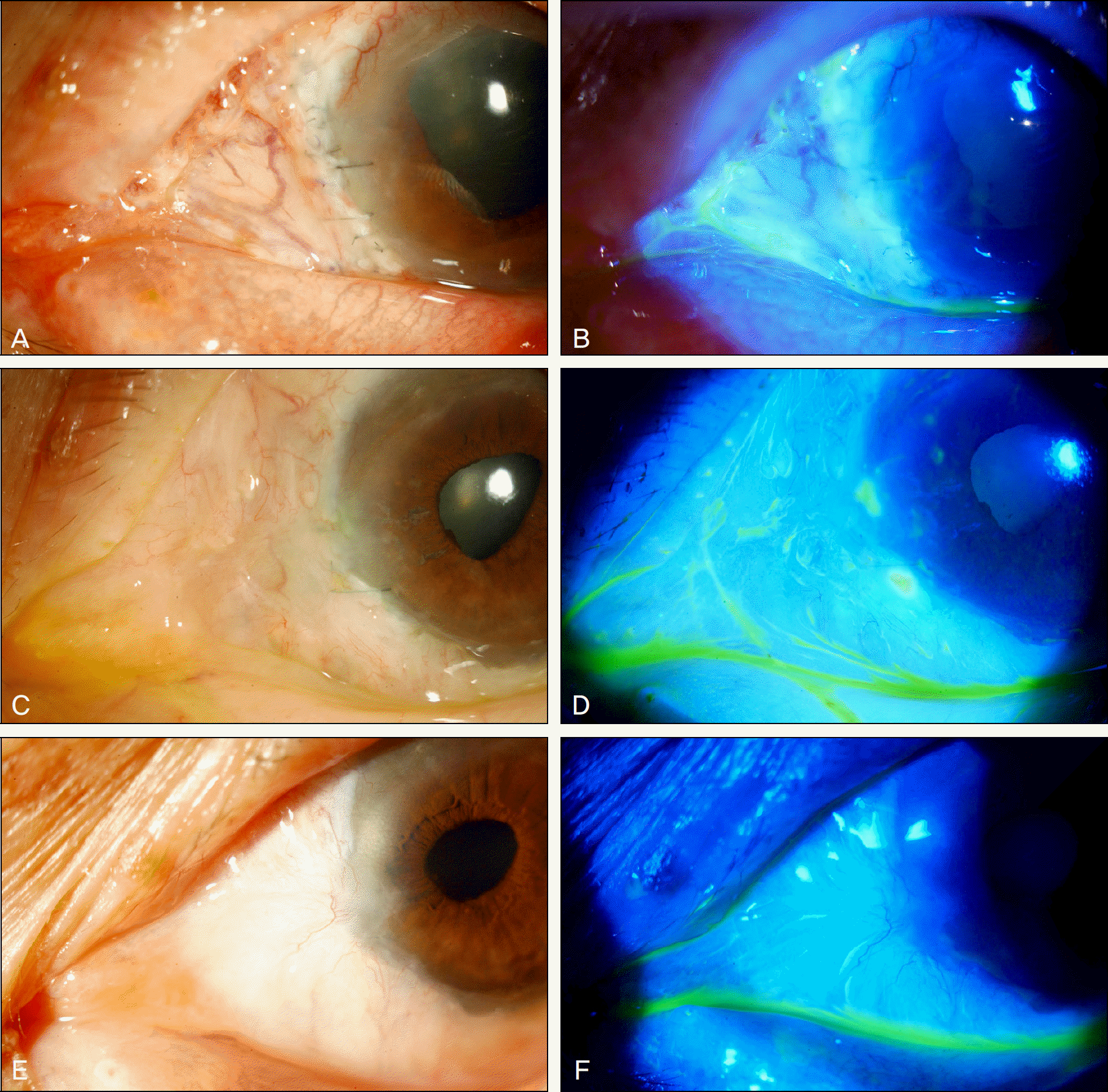
Figure 2.
The photographs show postoperative recovery after the autologous tragal perichondrium graft in refractory necrotizing scleritis patient. (A, B) One week after the surgery, vascularization has developed over the grafted perichondrium, and grafted tissue has been epithelized showing no defects on fluorescein staining. (C, D) After 3 months, conjunctivalization has started over the graft. (E, F) One year after the tragal perichondrium graft, conjunctivalization has been completed, and there was no complication.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download