Abstract
Purpose
To investigate the efficacy of an amniotic membrane contact lens on corneal epithelial wound healing.
Methods
We made a model with a corneal epithelial wound by applying 6 mm round filter paper soaked with 1 N NaOHonto the central cornea in 24 eyes of 12 rabbits. The rabbits were divided into three groups: AMCL (amniotic membrane contact lens), T-AMT (temporary amniotic membrane transplantation) and the control group. We evaluated corneal wound healing every postoperative day using a digital photo slitlamp and fluorescein dye. The corneas were harvested for histopathologic studies after seven days and analyzed with hematoxylin-eosin (H & E) stain and TUNEL staining.
Results
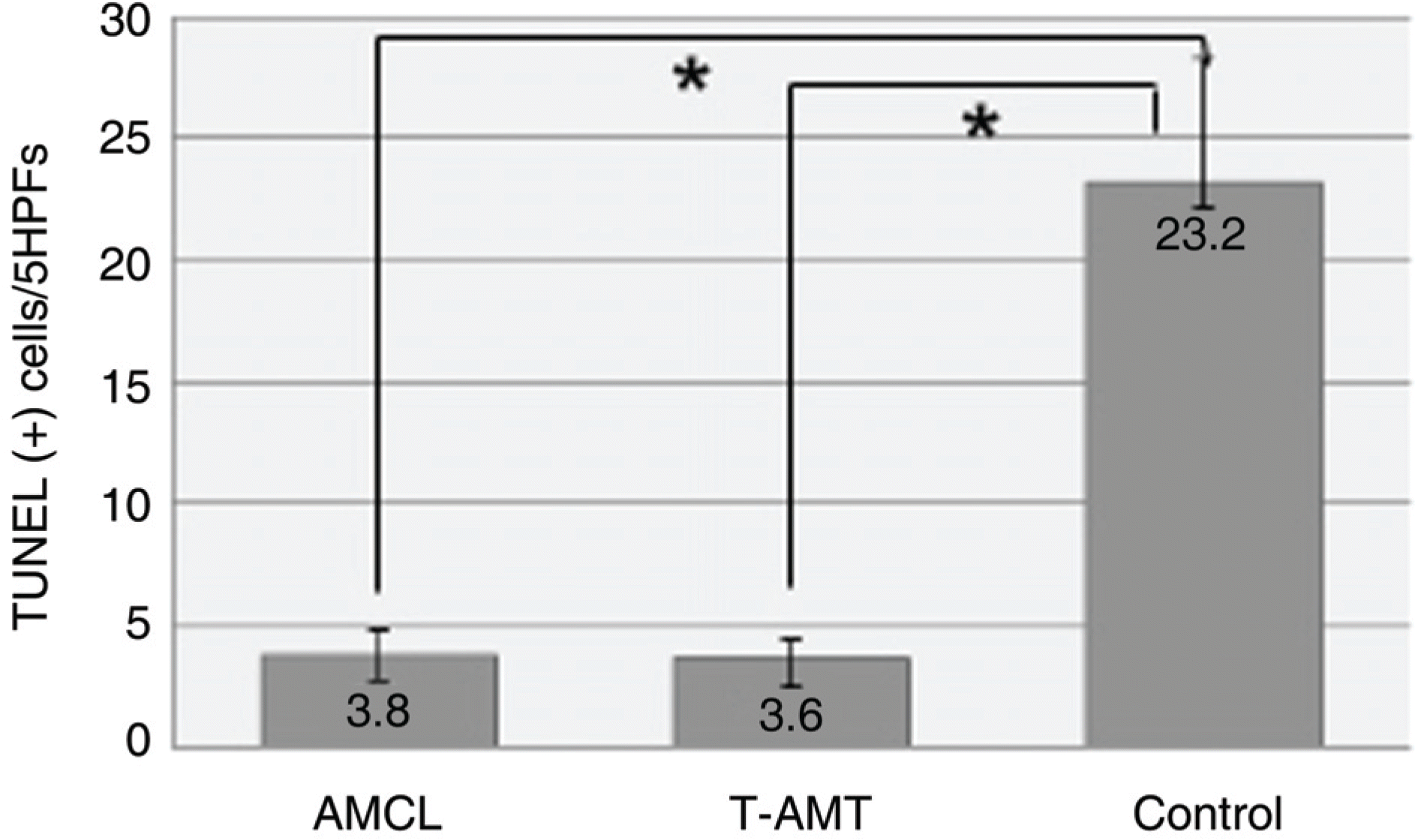
The average wound healing time was similar between the amniotic membrane contact lens and the temporary amniotic membrane transplantation group. The number of the infiltrated PMNs (polymorphonuclear cells) was 8.8±2.58, 8.6±2.19 and 48.6±7.12 in the AMCL, T-AMT and control groups, respectively. Apoptotic keratocytes were 3.8±1.1, 3.6±1.09 and 23.2±5.06 in the AMCL, T-AMT and control groups, respectively. In the AMCL and T-AMT groups, the number of infiltrated PMNs and apoptotic keratocytes were significantly less than those the control group (p<0.05). There were not significant differences in the number of PMNs and apoptotic cells in the AMCL and the T-AMT groups.
References
1. Choi YS, Kim J-Y, Wee WR. Effect of the application of human amniotic membrane rabbit corneal wound healing after excimer laser photorefractive keratectomy. Cornea. 1998; 17:389–95.
2. Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997; 123:303–12.

3. Chun DH, Jeon SL, Lee J-Y, Choi TH. The effect of amniotic membrane transplantation on corneal epithelial cell proliferation. J Korean Ophthalmol Soc. 2002; 43:1746–57.
4. Taylor RJ, Wang MX. Rate of reepithelialization following amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 1998; 39:S1038.
5. Fournier JH, McLachlan DL. Ocular surface reconstruction using amniotic membrane allograft for severe surface disorders in chemical burns: case report and review of the literature. Int Surg. 2005; 90:45–7.
6. Jain S, Rastogi A. Evaluation of the outcome of amniotic membrane transplantation for ocular surface reconstruction in symblepharon. Eye. 2004; 18:1251–7.

7. John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am. 2003; 16:43–65.
8. Barabino S, Rolando M, Bentivoglio G, et al. Role of amniotic membrane transplantation for conjunctival reconstruction in ocular-cicatricial pemphigoid. Ophthalmology. 2003; 110:474–80.

9. Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002; 109:1285–90.
10. Tseng SC, Prabhasawat P, Lee SH. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997; 124:765–74.

11. Meller D, Tseng SC. In vitro conjunctival epithelial differentiation on preserved human amniotic membrane. Invest Ophthalmol Vis Sci. 1998; 39:S428.
12. Tseng SC, Prabhasawat P, Barton K. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998; 116:431–41.

13. Taylor RJ, Wang MX. Rate of reepithelialization following amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 1998; 39:S1038.
14. Kim JS, Kim JC, Na BK, et al. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000; 70:329–37.

15. Buultmann S, You L, Spandau U, et al. Amniotic membrane down-regulate chemokine expression in human keratocytes. Invest Ophthalmol Vis Sci. 1999; 40:S3044.
16. Tseng SC, Li DQ, Ma X. Downregulation of TGF-b1, b2, b3, and TGF-b receptor II expression in human corneal fibroblasts by amniotic membrane. Invest Ophthalmol Vis Sci. 1998; 39:S428.
17. Rennekampff H-O, Dohrmann P, Fory R, Fandrich F. Evaluation of amniotic membrane as a adhesion prophylaxis in a novel surgical gastroschisis model. J Invest Surg. 1994; 7:187–93.
18. Chin JR, Murphy G, Werb Z. Stromelsin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. J Biol Chem. 1985; 264:1367–76.
19. Dogru M, Tsubota K. Current concepts in ocular surface reconstruction. Semin Ophthalmol. 2005; 20:75–93.

20. SIjiri S, Kobayashi A, Sugiyama K, Tseng SC. Evaluation of visual acuity and color vision in normal human eyes with a sutureless temporary amniotic membrane patch. Am J Ophthalmol. 2007; 144:938–42.
Figure 2.
Photograph of fluorescein dye staining shows corneal epithelial defect after alkali burn (A). Transplanted amniotic membrane contact lens (AMCL) is in place (B). Temporarily transplantated amniotic membrane (T-AMT) in place (C).

Figure 3.
Histopathologic findings of rabbit cornea with hematoxylin and eosin staining. Original magnification; ×200. Mild infiltration of inflammatory cells was detected in AMCL and T-AMT group (A and B). Infiltration of many inflammatory cells was found in the stroma in the control group (C). No definite differences are found between AMCL and T-AMT group.
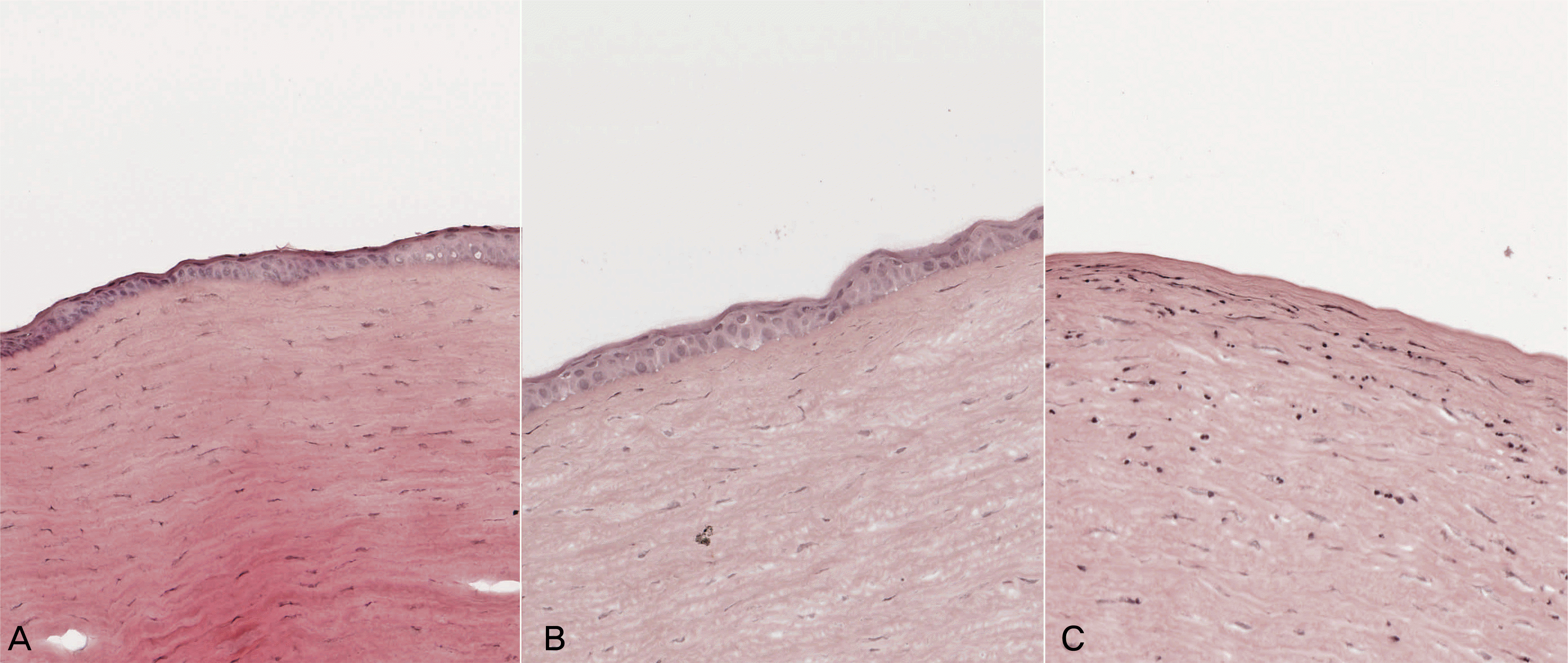
Figure 4.
Comparison of PMNs infiltration in the stroma. Infiltration of many inflammatory cells was detected in the control group. There was no significant difference between AMCL and T-AMT group (* p<0.05). PMNs= polymorphonuclear cells; HPF=high power field.

Figure 5.
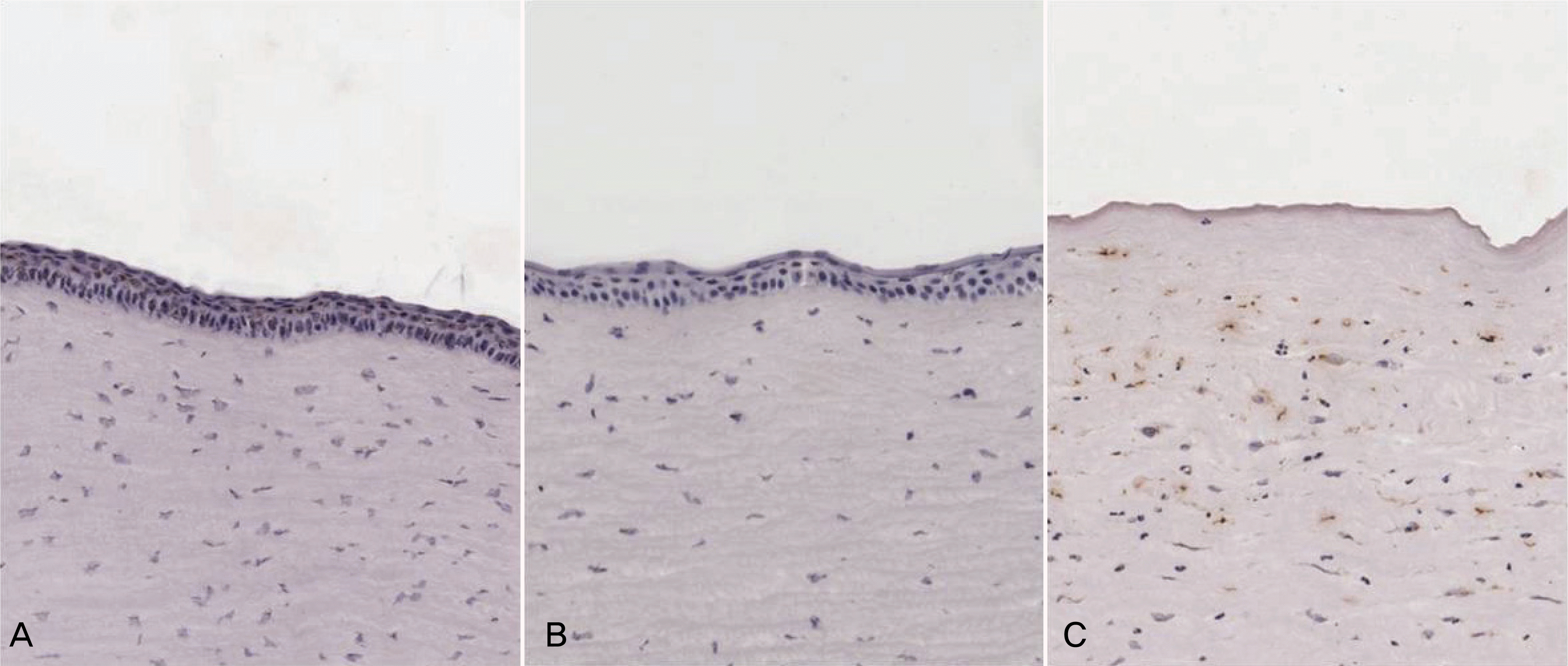
Histopathologic findings of rabbit cornea with TUNEL stain. Original magnification, ×200. The lesser TUNEL positive cells were detected in the anterior corneal stroma in AMCL and T-AMT group (A and B). More TUNEL positive cells were seen in the control group (C). There was no significant difference in the number of apoptotic cells between AMCL and T-AMT group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download