Abstract
Purpose
To investigate the role of L-ascorbic acid (LAA) on the survival and its association with nitric oxide (NO) production against hydrogen peroxide-induced oxidative stress in trabecular meshwork (TM) cells.
Methods
Primarily cultured human TM cells were exposed to hydrogen peroxide; 10, 100 µM for 2 days, or 1 mM single exposure with or without co-exposure of LAA. Cellular survival and nitrite production were assessed with MTT and Griess assays, respectively. Flow cytometry using annexin/PI double staining was performed to evaluate apoptosis.
Results
Hydrogen peroxide decreased cellular survival significantly in a dose-dependent manner accompanied with decreased NO production. However cellular survival and NO production were increased significantly with co-exposure of LAA. Cellular survival and NO were highly correlated. Flow cytometric analysis revealed that LAA inhibited hydrogen peroxide-induced apoptosis.
References
1. Becker B. Chemical composition of human aqueous humor. Effects of acetazolamide. AMA Arch Ophthalmol. 1957; 57:793–800.
2. Erb C, Nau-Staudt K, Flammer J, Nau W. Ascorbic acid as a free radical scavenger in porcine and bovine aqueous humour. Ophthalmic Res. 2004; 36:38–42.

3. Heller R, Münscher-Pailug F, Gräbner R, Till U. L-ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J Biol Chem. 1999; 274:8254–60.

4. Kim JW. Ascorbic acid enhances nitric oxide production in cultured trabecular meshwork cell. Korean J Ophthalmol. 2005; 19:227–32.
5. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.
6. Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994; 63:175–95.

7. Brüne B, von Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998; 351:261–72.

8. Beck K-F, Eberhardt W, Frank S. . Inducible NO synthase: Role in cell signaling. J Exp Biol. 1999; 202:645–53.
9. Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway. Invest Ophthalmol Vis Sci. 1995; 36:1765–73.
10. Geyer O, Podos SM, Mittag T. Nitric oxide synthase activity in tissues of the bovine eyes. Graefes Arch Clin Exp Ophthalmol. 1997; 235:786–93.
11. Meyer P, Champion C, Schlotzer-Schrehardt U. . Localization of nitric oxide synthase isoforms in porcine ocular tissues. Curr Eye Res. 1999; 18:375–80.

12. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and ocular facility in monkeys. Exp Eye Res. 1994; 58:99–105.
13. Wana RF, Podos SM. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995; 60:337–9.
14. Nathanson JA, McKee M. Alteration of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995; 36:1774–84.
15. Matsuo T. Basal nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–5.

16. Green LC, Wagner DA, Glogoski J. . Analysis of nitrate, nitrite and 15N nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.
17. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–9.
18. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–78.
19. Ross R. Atherosclerosis-an inflammatory disease. N Eng J Med. 1999; 340:115–26.
20. May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000; 28:1421–9.
21. Huang A, Vita JA, Venema RC, Keaney JF Jr. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000; 275:17399–406.

22. Heller R, Unbehaun A, Schnellenberg B. . L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001; 276:40–7.

23. Padgaonkar V, Giblin FJ, Leverenz V. . Studies of H2O2-induced effects on cultured bovine trabecular meshwork cells. J Glaucoma. 1994; 3:123–31.

24. Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthase: structure, function, and inhibition. Biochem J. 2001; 357:593–615.
25. Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005; 25:29–38.

26. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–79.

27. Millard CB, Tripathi BJ, Tripathi RC. Age-related changes in protein profiles of the normal human trabecular meshwork. Exp Eye Res. 1987; 45:623–31.

28. Schchschabel DO, Binninger E. Aging of trabecular meshwork cells of the human eye in vitro. Z Gerontol. 1990; 23:133–5.
29. Horstmann HJ, Rohen JW, Sames K. Age-related changes in the composition of proteins in the trabecular meshwork of the human eye. Mech Ageing Dev. 1983; 21:121–36.

30. Vasa M, Breitschopf K, Zeiher AM, Dimmeler S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res. 2000; 87:540–2.

31. Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from ocular tissue: the trabecular meshwork. J Cell Physiol. 1999; 180:182–9.
32. Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004; 117:2417–26.

33. von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci. 2000; 908:99–110.

34. Wei YH. Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med. 1998; 217:53–63.

35. Furumoto K, Inoue E, Nagao N. . Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998; 63:935–48.

Figure 1
. Effect of L-ascorbic acid on the survival of cultured trabecular meshwork cells. L-ascorbic acid increased cellular survival in a dose-dependent manner. (* p<0.05)
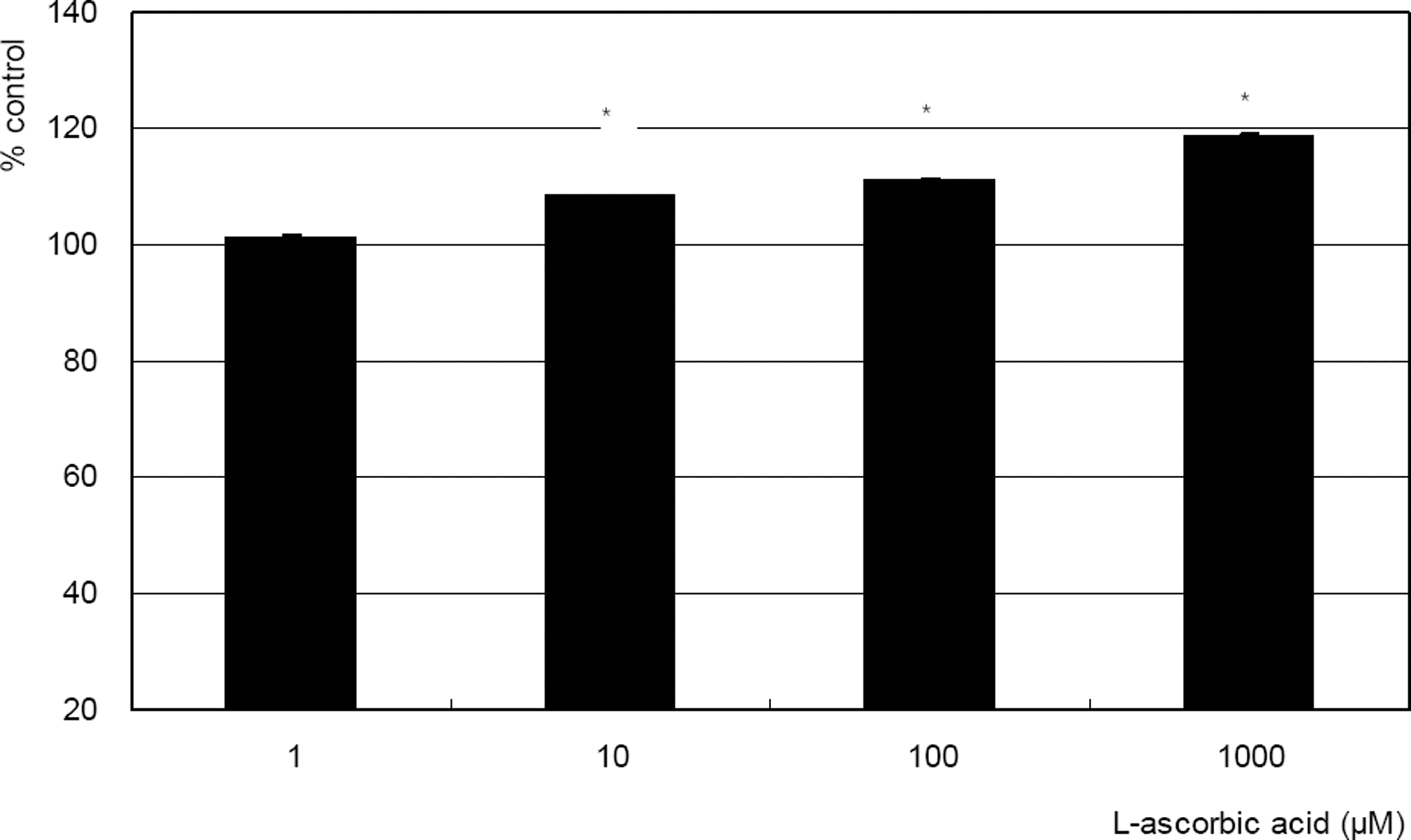
Figure 2
. Effect of hydrogen peroxide on the survival of cultured TM cells exposed to 10, 100 µM for 2 days, or 1 mM single exposure. Hydrogen peroxide decreased cellular survival significantly in both conditions. (* p<0.05)
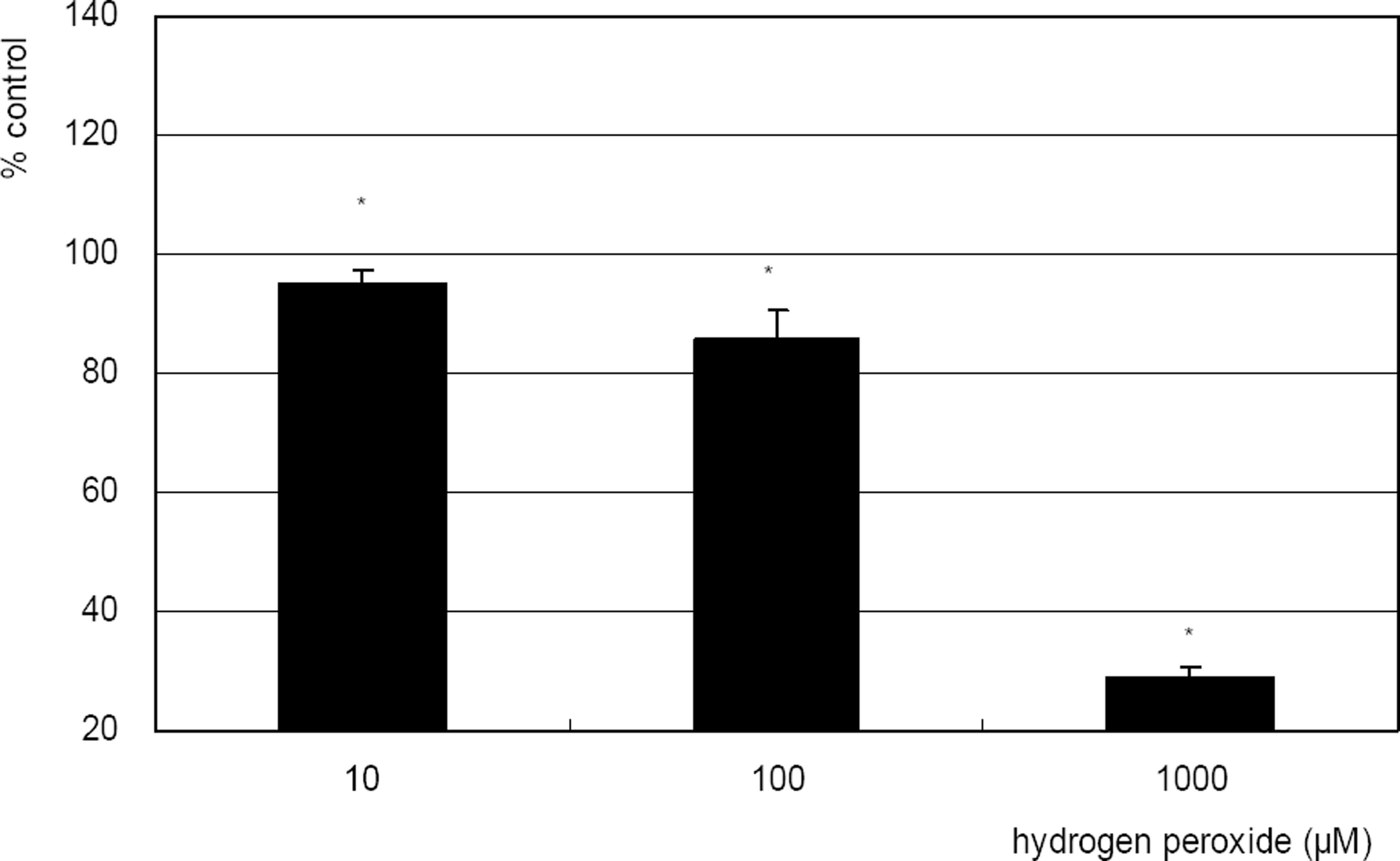
Figure 3
. L-ascorbic acid increased survival of cultured trabecular meshwork cells exposed to 10, or 100 µM hydrogen peroxide. (* p<0.05)
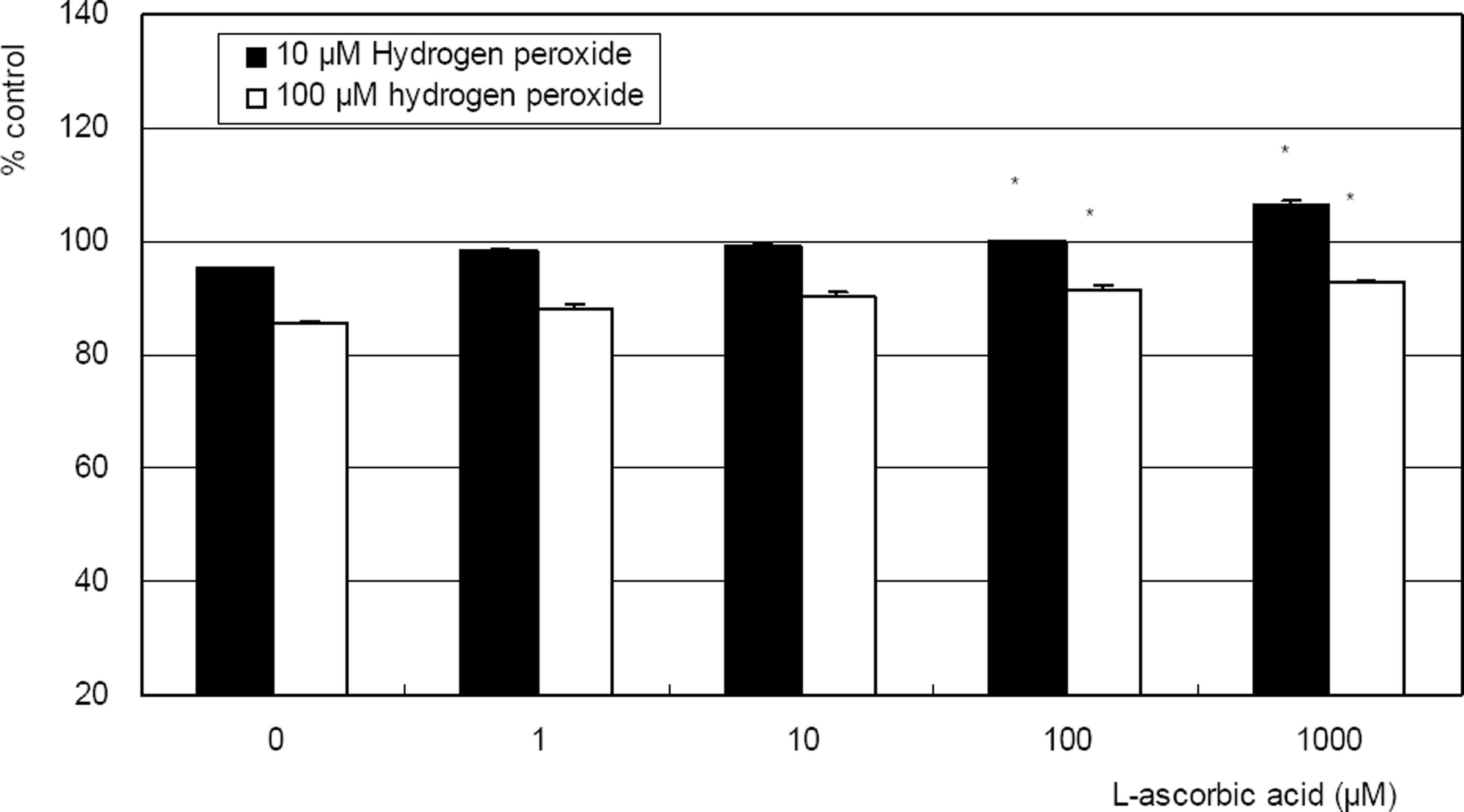
Figure 4
. Comparison of the effects of 0.5 mM L-NAME, 100 µM L-ascorbic acid (LAA), or 100 µM hydrogen peroxide on the survival of cultured trabecular meshwork cells. (* p<0.05)
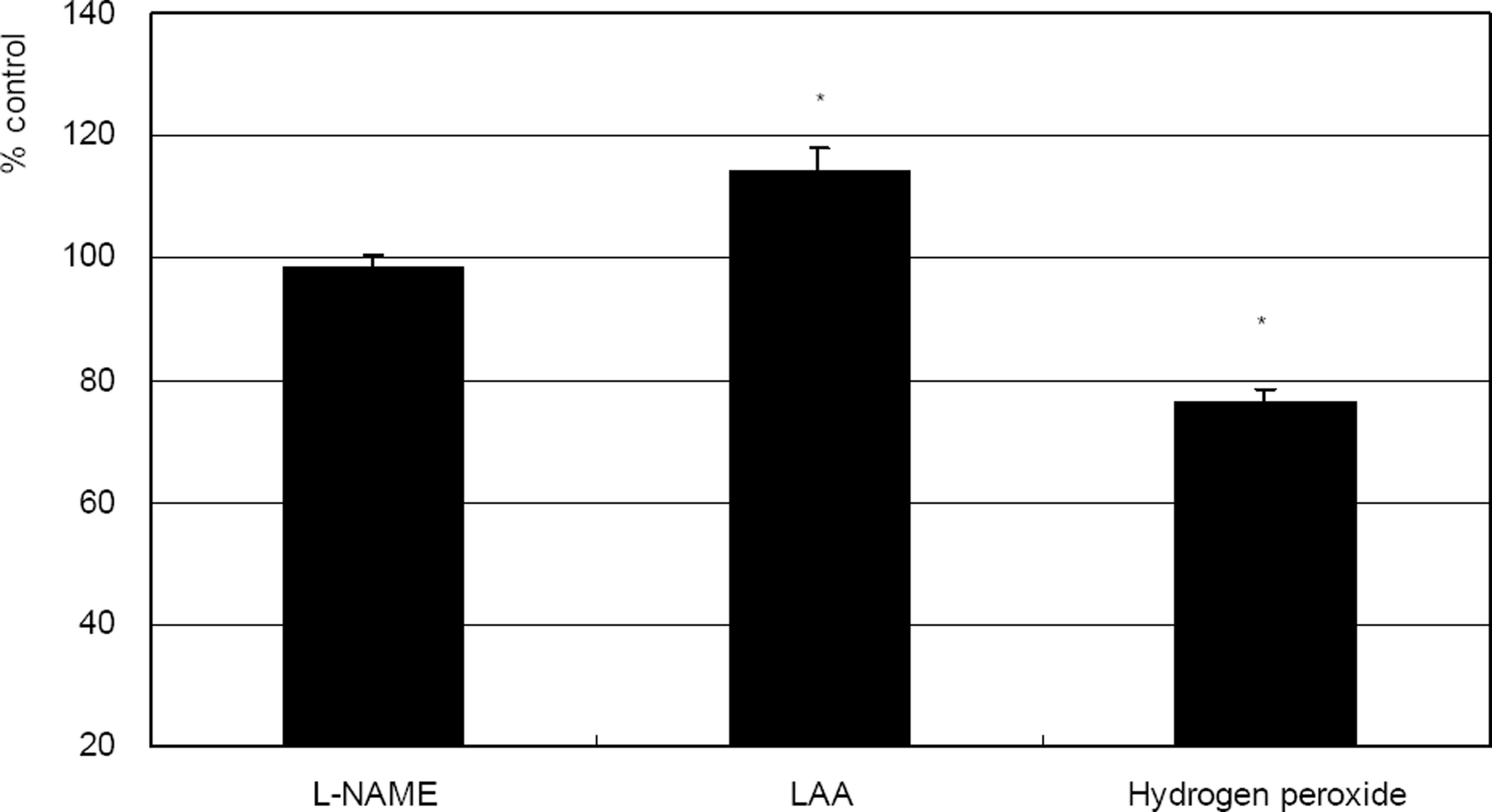
Figure 5
. Effect of L-ascorbic acid (LAA) on the production of nitric oxide in cultured trabecular meshwork cells. LAA increased nitric oxide production in a dose-dependent manner. (* p<0.05)
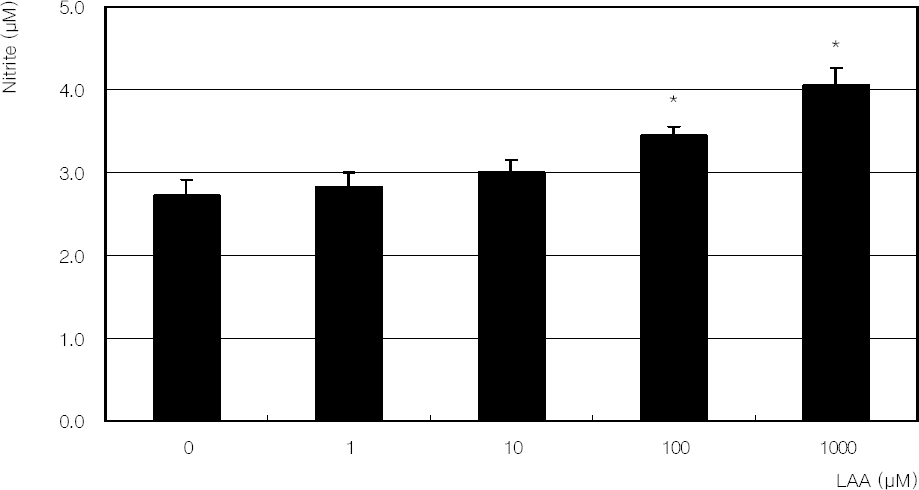
Figure 6
. Effect of hydrogen peroxide on the production of nitric oxide in cultured trabecular meshwork cells. Hydrogen peroxide decreased nitric oxide production significantly. (* p<0.05)
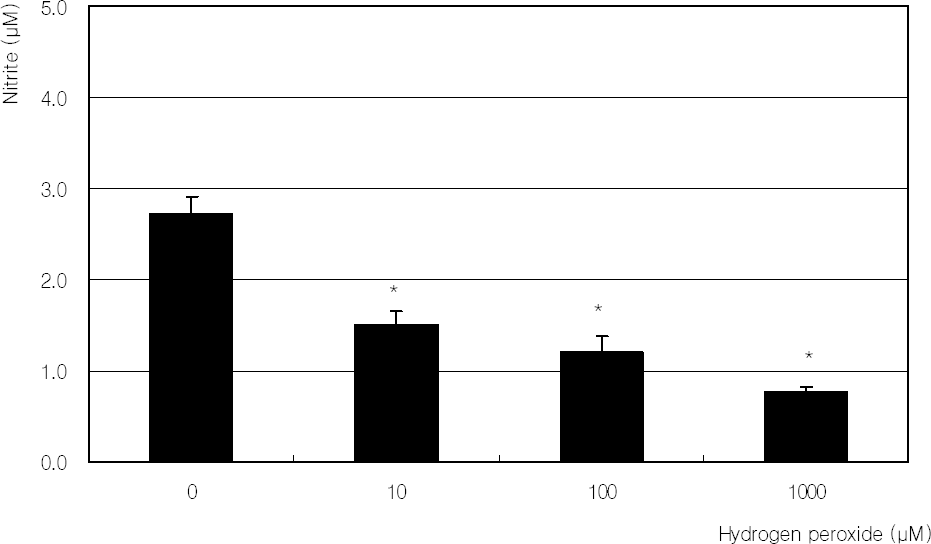
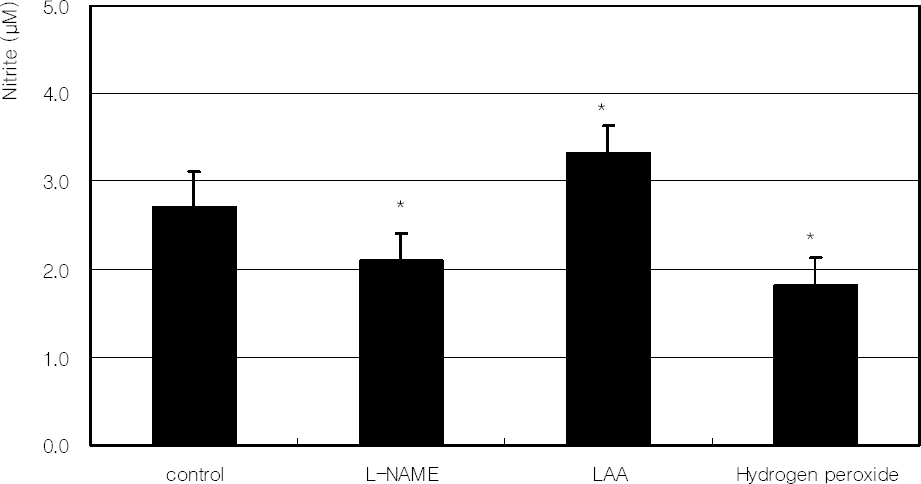
Figure 7
. Effect of L-NAME, 100 µM L-ascorbic acid (LAA), or 100 µM hydrogen peroxide on the production of nitric oxide. (* p<0.05)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



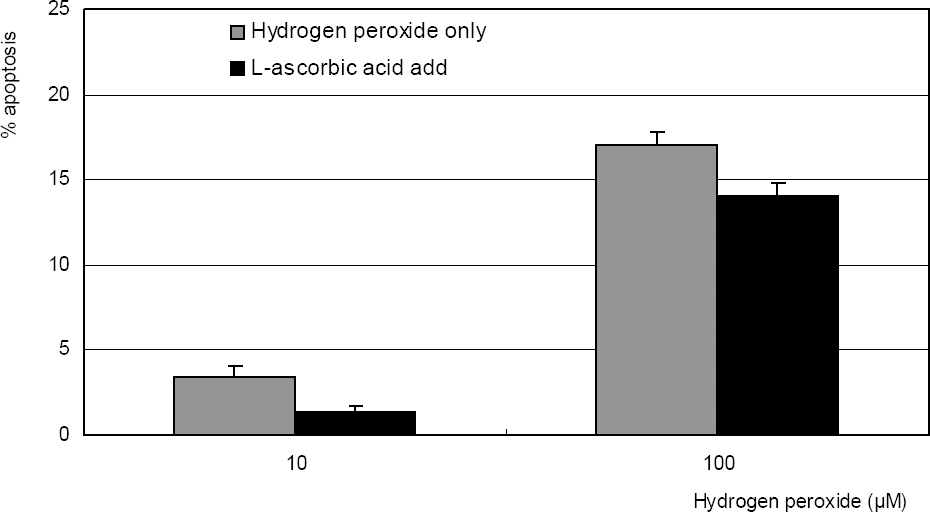
 XML Download
XML Download