Abstract
A 57-year-old woman scheduled for cochlear implant removal exhibited preoperative electrocardiographic findings of early repolarization (ER). Four episodes of transient ST segment elevations during surgery raised suspicion for vasospastic angina (VA). In the post-anesthetic care unit, the patient complained of chest discomfort and received sublingual nitroglycerin with uncertain effect. The patient refused to proceed with postoperative invasive coronary angiography, resulting in inconclusive diagnosis. Intraoperative circumstances limit the diagnosis of VA, which emphasizes the need for further testing to confirm the diagnosis. When VA is suspected in patients with underlying ER, it is reasonable to consider invasive examination to establish the diagnosis and prevent recurrence of VA. If ST changes are observed during surgery in patients with preoperative ER, careful monitoring is recommended. Due to general anesthesia, the absence of patient symptoms limits the definitive diagnosis of those with suspected VA. Therefore, additional postoperative surveillance is recommended.
Early repolarization (ER) is an electrocardiographic finding of QRS-ST junction elevation (> 0.1 mV) in two adjacent leads, demonstrated as either QRS complex notching or slurring [1]. The prevalence of ER ranges up to 13% in the general population. ER has generally been regarded to be an innocuous finding in a healthy population [2]. Recent studies, however, have demonstrated an association between ER and an increased risk for serious arrhythmias and death [345]. ER is a predictor of vasospastic angina (VA) and an indicator of multivessel vasospasm [6]. The present article describes a case involving a patient exhibiting a preoperative ER pattern who experienced intraoperative transient―yet severe―ST amplitude changes and postoperative chest discomfort, which raised suspicion for VA, which emphasizes the need for perioperative surveillance.
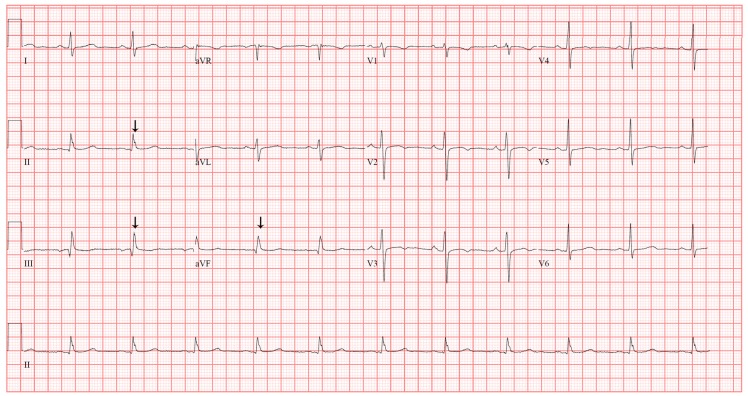
A 57-year-old woman (height 150.7 cm; weight 71.3 kg; body mass index 31.3 kg/m2) with a history of noninsulin-dependent diabetes mellitus and chronic hypertension complained of posterior auricular pain, which began 10 months previously. She underwent multiple surgeries on her left ear due to chronic otitis media, including a subtotal petrosectomy and a cochlear implant one year ago. Without improvement of her pain with wound debridement and antibiotic medication, cochlear implant removal was planned. Preoperative 12-lead electrocardiography (ECG) revealed a slurred QRS complex and J point elevation in leads II, III, and aVF (Fig. 1), which was not different from the ECG performed a year ago in a previous pre-anesthetic work-up. Preoperative laboratory examinations and two-dimensional echocardiogram revealed no abnormalities.
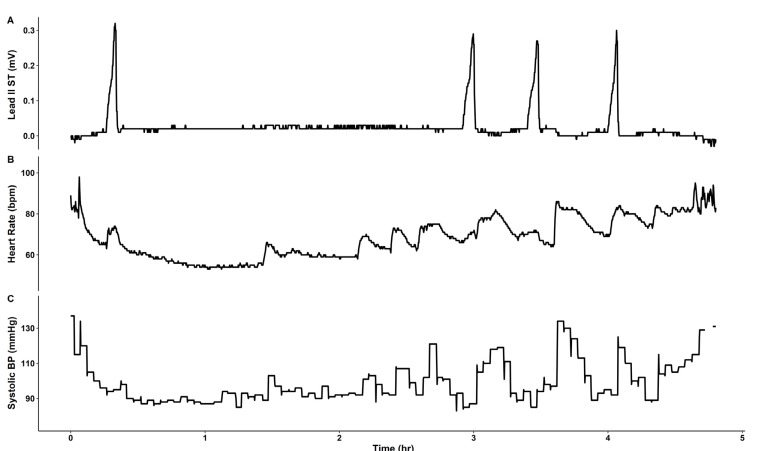
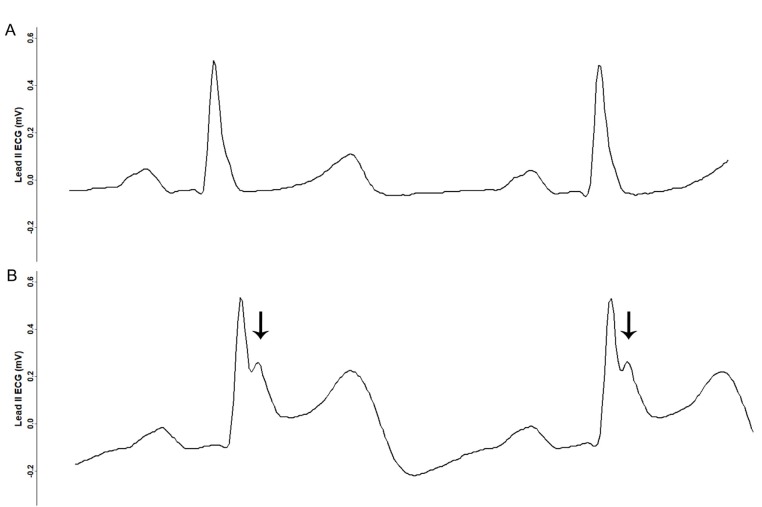
The patient was monitored using pulse oximetry, automated blood pressure measurement, capnography, and three-lead ECG. Anesthesia was induced using propofol 140 mg, sevoflurane 6% vol, and rocuronium 40 mg, and tracheal intubation was performed. Anesthesia was maintained with sevoflurane 1–2% vol, 1 L/min N2O, and 1 L/min O2. During the 4 h procedure, four episodes of transient ST segment elevation in lead II were observed, with maximum ST segment amplitude of 0.3 mV (Fig. 2, 3). The ECG pattern changed from a slurred to a notched appearance, with a J point elevation changing from 0.1 mV to 0.2 mV (Fig. 3). Each elevation lasted < 5 min without hemodynamic instability. Coronary artery spasm or benign ECG findings of the ER pattern were considered. At the third episode of ST segment change, cardiac markers were noted, and both troponin-I (0.006 ng/ml) and creatine kinase-MB (0.4 ng/ml) were within normal ranges. Given that ECG changes were temporal and without hemodynamic change, and laboratory investigations were normal, diagnosis could not be established and, thus, no immediate treatment was pursued.
After uneventful extubation, the patient was transferred to the post-anesthetic care unit (PACU). However, she complained of chest discomfort. On three-lead ECG monitoring, ST-segment elevation was not observed. For detailed evaluation, a 12-lead ECG was performed, but was no different from the preoperative ECG. Although vital signs and cardiac enzyme levels, including troponin-I (0.006 ng/ml) and creatine kinase-MB (0.2 ng/ml), were within normal range, VA was considered based on the intraoperative transient ST changes and symptoms in the PACU. Therefore, sublingual nitroglycerin was administered; however, its effect was unclear, and the chest discomfort lasted for approximately 1 h. After symptoms subsided, the patient was transferred to the general ward because vital signs were stable throughout the entire period and laboratory findings were within normal ranges. On follow-up consultation with the cardiology department, the possibility of coronary artery spasm was raised. However, the patient refused further work-ups, including echocardiography and coronary angiogram. The patient was discharged 10 days later without further complaints of chest symptoms and, thus, the cause of ST elevations remained unclear.
ER is defined on ECG pattern as QRS-ST junction, also known as J point elevation (> 0.1 mV) in two adjacent leads, demonstrated as either QRS complex notching or slurring [1]. The prevalence of ER ranges up to 13% in the general population. ER has generally been regarded to be an innocuous finding in a healthy population [2]. However, some recent clinical studies reported that ER is associated with an increased risk for serious arrhythmias and death [345] and increased cardiac events in low-risk surgical patients one year after surgery [7]. Baseline ER is a predictor of VA and an indicator of multi-vessel vasospasm [6].
VA is a transient myocardial ischemia resulting in chest pain and ST-segment elevation, and can result in fatal arrhythmias or sudden cardiac death. VA is possibly caused by coronary artery spasm [8]. The Coronary Vasomotion Disorders International Study Group diagnostic criteria for VA includes nitrate-responsive angina during spontaneous episodes, transient ischemic ECG changes, such as ST segment elevation or depression, and the presence of coronary artery spasm involving the injection of a provocative agent to reproduce usual chest pain, ECG changes, and vasoconstriction on angiography [9]. Evident nitrate-responsive angina with transient ECG changes, or coronary artery spasm manifestation, fulfill the criteria for diagnosis of definitive VA and, thus, provocative coronary artery spasm testing is required when documentation of ECG changes is unavailable [9]. Considering the possible relationship between the episodes of intraoperative ST segment elevations and chest discomfort in the patient while in the PACU, we suspected VA and administered sublingual nitroglycerin. For VA, calcium channel blockers, as well as nitrate, are considered among the standard treatments. However, the prevalence of refractory VA is approximately 5–30% [10], and further examinations should be considered to differentiate VA from benign ER.
Intraoperative circumstances may limit the diagnosis of VA. The classic symptoms of chest pain are often absent during surgery and masked postoperatively by intensive pain control [11]. Electrocardiographic differential diagnosis is limited during surgery because the three-electrode system does not display multi-lead monitoring or precordial leads and, is thus, inadequate for ST-segment monitoring. These restrictions emphasize that perioperative suspicions of VA should undergo an examination to confirm the diagnosis. A limitation of the current case was that patient did not undergo further cardiac evaluation to confirm VA and, therefore, a definitive diagnosis could not be made. Furthermore, preoperative invasive coronary angiography was not performed because it is generally not a routine examination in patients without a history of chest pain or cardiac disease [9]. However, within the 3-month follow-up, the patient did not report any cardiac symptoms.
In the current case, suspicion of VA was raised by ST-segment elevations during surgery followed by chest discomfort during recovery. Lack of patient symptoms during general anesthesia limits the differential diagnosis, and raises the need for additional postoperative surveillance to establish the appropriate diagnosis and prevent the recurrence of VA.
References
1. Macfarlane PW, Antzelevitch C, Haissaguerre M, Huikuri HV, Potse M, Rosso R, et al. The Early Repolarization Pattern: A Consensus Paper. J Am Coll Cardiol. 2015; 66:470–477. PMID: 26205599.
2. Benito B, Guasch E, Rivard L, Nattel S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol. 2010; 56:1177–1186. PMID: 20883924.
3. Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008; 52:1231–1238. PMID: 18926326.
4. Sinner MF, Reinhard W, Muller M, Beckmann BM, Martens E, Perz S, et al. Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: a population-based prospective cohort study (MONICA/KORA). PLoS Med. 2010; 7:e1000314. PMID: 20668657.

5. Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S, Kerola T, et al. Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome. Circulation. 2011; 123:2666–2673. PMID: 21632493.
6. Inamura Y, Nishizaki M, Shimizu M, Fujii H, Yamawake N, Suzuki M, et al. Early repolarization and positive T-wave alternans as risk markers for life-threatening arrhythmias in patients with vasospastic angina. Int J Cardiol. 2015; 196:7–13. PMID: 26070177.

7. Ota C, Shiono SN, Fujino Y, Kamibayashi T, Hayashi Y. Prevalence and Prognostic Value of Early Repolarization in Low Risk Surgical Patients. Biomed Res Int. 2015; 2015:309260. PMID: 26266254.

8. Oh CM, Oh J, Shin DH, Hwang HJ, Kim BK, Pak HN, et al. Early repolarization pattern predicts cardiac death and fatal arrhythmia in patients with vasospastic angina. Int J Cardiol. 2013; 167:1181–1187. PMID: 22483625.

9. Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017; 38:2565–2568. PMID: 26245334.

10. Rajiv A, Manjunath CN. Refractory vasospastic angina. J Cardiovasc Dis Res. 2015; 6:189–191.
11. Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012; 307:2295–2304. PMID: 22706835.

Fig. 1
12-lead surface electrocardiography (25 mm/s, 10 mm/mV) demonstrating early repolarization pattern in leads II, III and aVF. Arrows indicate slurred QRS complex and J point.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download