Abstract
Background
Various techniques have been introduced to decrease complications during nasotracheal intubation. A common practice is to use nasal packing with a cotton stick and 0.01% epinephrine jelly. However, this procedure can be painful to patients and can damage the nasal mucosa. Xylometazoline spray can induce effective vasoconstriction of the nasal mucosa without direct nasal trauma. In this study, we aimed to compare the efficacy of these two methods.
Methods
Patients were randomly allocated into two groups (n = 40 each): xylometazoline spray group or epinephrine packing group. After the induction of general anesthesia, patients allocated to the xylometazoline spray group were treated with xylometazoline spray to induce nasal cavity mucosa vasoconstriction, and the epinephrine packing group was treated with nasal packing with two cotton sticks and 0.01% epinephrine jelly. The number of attempts to insert the endotracheal tube into the nasopharynx, the degree of difficulty during insertion, and bleeding during bronchoscopy were recorded. An anesthesiologist, blinded to the intubation method, estimated the severity of epistaxis 5 min after intubation and postoperative complications.
Results
No significant intergroup difference was observed in navigability (P = 0.465). The xylometazoline spray group showed significantly less epistaxis during intubation (P = 0.02). However, no differences were observed in epistaxis 5 min after intubation or postoperative epistaxis (P = 0.201). No inter-group differences were observed in complications related to nasal intubation and nasal pain.
Nasotracheal intubation (NTI) is frequently performed for oral and maxillofacial surgeries [1]. However, the nasal cavity is very narrow and has a large distribution of blood vessels; hence, the mucosa of the nasal cavity is fragile. Severe complications can occur during NTI, including bleeding, laceration of the mucosa, and trauma to the nasopharyngeal airway [2345]. To decrease these complications, various methods have been developed, including lubrication with a water-soluble jelly, atraumatic tube design, use of topical vasoconstrictors, thermo-softening of the endotracheal tube (ETT), and obturation of the tube tip with a balloon or esophageal stethoscope [6789101112]. Of these methods, topical vasoconstrictors (cocaine, epinephrine, phenylephrine, xylometazoline, and oxymetazoline) have been used to decrease epistaxis and have shown similar reduction in the incidence of epistaxis. A conventional method using vasoconstrictors utilizes nasal packing with cotton swabs moistened with a vasoconstrictor and water-soluble jelly mixture. However, this method may induce discomfort or pain even with the use of topical anesthetics, to the point that some patients would refuse to cooperate. Thus, we usually initiate nasal packing after the induction of general anesthesia [13]. We usually insert the cotton swab blindly and cannot guarantee the packing site is optimal for nasal intubation. For safety and convenience, we investigated other options of topical vasoconstrictor therapy with the following conditions: easy to prepare, no need of needles or other instruments that might harm the patient or physician, no insertion of cotton swabs into the nasal cavity, and no need for an anesthesiologist to dilute high-concentration vasoconstrictors like epinephrine and phenylephrine in the operating room. A previous study reported the successful use of 0.1% xylometazoline nasal drip [13]. We assumed that 0.1% xylometazoline spray, which has been commonly used to decrease nasal congestion, may have a similar effect and tried to determine whether this method was effective [131415]. The primary aim of this study was to investigate the efficacy of intranasal pretreatment with xylometazoline (0.1%) spray over cotton swab packing with epinephrine and a water-soluble jelly mixture [16].
The study protocol was approved by the institutional review board (IRB) of Dankook University Hospital (DKUH IRB No. 2016-11-006). Informed consent was confirmed by the IRB.
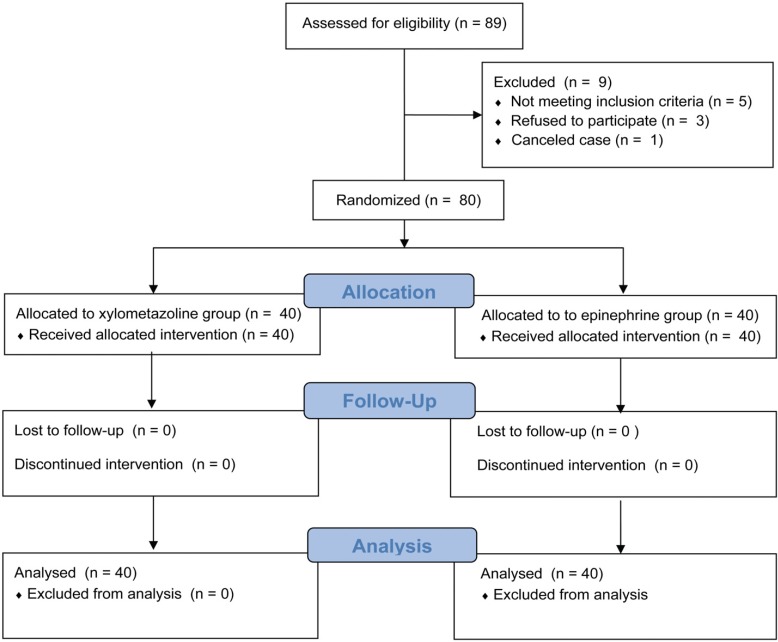
Written informed consent was obtained from 80adult patients with American Society of Anesthesiologists (ASA) classes I and III, who underwent elective oral and maxillofacial surgery requiring NTI between January 2016 and July 2016. This was a prospective randomized study. The exclusion criteria were age under 18 years, history of nasal abnormality (e.g., nasal trauma, surgery, obstruction, and polyp), history of repeated epistaxis, drug use (oral decongestants, non-steroidal anti-inflammatory drugs and anti-histamine drugs, and anticoagulation therapy), anticipated difficult airway management, and mental disorders. Patients were randomly assigned to one of two groups by using sealed envelopes that were opened just before the induction of anesthesia: xylometazoline spray (XS) group and epinephrine swab packing (EP) group (Fig. 1).
In the operating rooms, standard monitoring equipment was attached. Before the induction of anesthesia, we examined the nasal cavity by using a Shikani Optical Stylet (Clarus Medical, Minneapolis, MN, USA) and selected the wider side of the nostril for intubation. If the nostrils were considered equally patent, the right nostril (which has been reported to bleed less than the left nostril does) was selected [1].
Patients in the XS group were asked to aspirate 0.1% xylometazoline spray (Otrivine 0.1% Nasal Spray) puffing two times (with full inspiration via the nostril). Patients in the EP group used 0.9% normal saline spray instead of xylometazoline.
No premedication was administered. Both groups were given intravenous fentanyl (1.5 µg/kg) and 100% oxygen for 1 min. Thereafter, lidocaine (40 mg), propofol (1.5 mg/kg), and rocuronium (0.6 mg/kg) were administered intravenously through a rapidly running infusion placed in the forearm. After confirming the loss of consciousness, two cotton swabs moistened with a water-soluble jelly (XS group) or a water-soluble jelly with epinephrine 1:10,000 (EP group) were inserted into the nasal cavity via the selected nostril. An anesthesiologist prepared the epinephrine jelly by mixing 1 ml water-soluble jelly with 0.1 ml of 1:1,000 epinephrine. Approximately 0.5 ml of epinephrine jelly was used each time. After mask ventilation with 100% oxygen with desflurane 8–10 vol% for 2 minutes, intubation was performed by the blinded anesthesiologist.
A lubricated Polar™ Preformed Tracheal Tube (Smith Medical, Keene, NH, USA) was used for NTI. A size of endotracheal tube (ETT) was selected by the following criteria: 7.0-mminternaldiameter tube for male adults and a 6.5-mm internal-diameter tube for adult females or adult males shorter than 165 cm in height. The ETT was gently inserted into the selected nostril towards the lower airway of nasal cavity. In this phase, if some resistance was felt, the ETT was withdrawn and reinserted with gentle cephalad tilting of the tube with or without counter-clockwise rotation. If resistance was felt again, reinsertion into the other nostril was attempted using the same method. After the placement of the ETT into the oropharynx, the ETT was inserted using conventional direct laryngoscopy with or without the aid of Magill forceps.
The degree of difficulty during ETT insertion into the nasopharynx, and bleeding during direct laryngoscopy were recorded by the anesthesiologist who performed the intubation. The degree of difficulty during ETT insertion was defined as follows: smooth, impinged, impossible, or nostril change. The degree of bleeding during bronchoscopy was estimated as follows: none to minimal—no bleeding, does not interfere with the laryngoscopic view, or blood-tinged ETT but no blood on the vocal cords or mouth floor; moderate—interferes with the laryngoscopic view, but is easy to confirm the laryngeal structure, with blood on the vocal cords and mouth floor; severe—hard to visualize the laryngeal structure without suction because of bleeding. The severity of epistaxis was estimated 5 min after intubation by another anesthesiologist who was blinded to the intubation method. Epistaxis after intubation was measured by pharyngeal aspiration using a 14-F, 50-cm-long suction catheter connected to a 2.5-m-longsuction tubing at a pressure of -100 mmHg [12]. The grade of epistaxis was measured according to the aspirated blood length (ABL) through the suction tube: none = no bleeding; mild = ABL < 50 cm; moderate = 50 cm < ABL < 300 cm; severe = ABL > 300 cm.
General anesthesia was continued with desflurane 6-7 vol% with intermittent injection of rocuronium after intubation. After the end of surgery, anesthetics were discontinued and extubation was performed after the reversal of muscle relaxation with atropine and neostigmine. The patient was interviewed about nasal complications, including nasal pain and persistent epistaxis by the blinded anesthesiologist just before discharge from the post-anesthesia care unit.
Data were analyzed using SPSS for Windows (Version 12.0, SPSS Inc., Chicago, IL, USA). We performed a pilot study, and the “none to minimal epistaxis rate” during intubation was 0.8 in the XS group and 0.5 in the EP group. Power analysis indicated that 36 patients per group would be enough to detect the difference of epistaxis during intubation with a power of 80% at the level of significance 0.05. We included 40 patients in each group to account for possible drop-outs.
Age, body weight, height, and severity of epistaxis were compared using paired t-tests, and sex, ASA class, intubation characteristics, and postoperative complications were tested using Fisher's exact test. P < 0.05 was considered statistically significant.
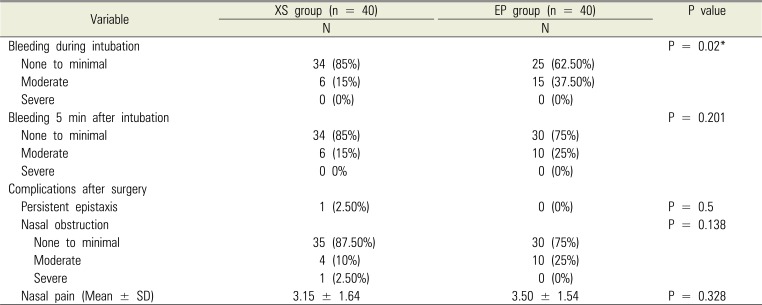
No statistically significant intergroup differences were observed in age, sex, height, body weight, and ASA class (Table 1). No significant intergroup difference was observed in navigability (Table 2). No intubation failure or nostril change was reported in either group (Table 2). The XS group showed significantly less epistaxis during intubation; however, no difference was observed in epistaxis 5 min after intubation and postoperative epistaxis. No intergroup difference was observed in complications related to nasal intubation and nasal pain (Table 3).
In this study, the overall incidence and severity of intubation-related nasal bleeding were similar in both groups. Thus, we can assume that xylometazoline spray treatment has a similar effect to conventional nasal packing with 0.01% epinephrine jelly.
Xylometazoline is an imidazole derivative designed to mimic the molecular shape of epinephrine [14]. Xylometazoline binds with alpha 1 and 2 adrenergic receptors and can induce vasoconstriction [14]. Xylometazoline 0.1% spray has been used to treat nasal congestion and has been proven safe, and it is currently being used without prescription [1517].
We considered other topical vasoconstriction treatment options other than epinephrine packing with a cotton swab because of its several disadvantages. First, we usually use two or three cotton swabs, and we cannot guarantee whether the epinephrine jelly would spread evenly enough to induce vasoconstriction through the “right” route of the nasal ETT. Second, we sometimes encountered patients with damage to the nasal mucosa and bleeding while inserting the cotton swab into the nasal cavity, especially when the procedure was performed by an inexperienced physician. Third, we cannot assume exactly how much of the epinephrine dose is delivered to the patient's nostril. Fourth, there is a risk of errors while preparing the vasoconstrictor. Son and Lee [18] reported a case of an accidental overdose of phenylephrine (nasal spray with 1.5 ml of 0.5% phenylephrine instead of 0.5 ml in a 13-year-old boy having a height of 1.65 m and weighing 76 kg), which resulted in pulmonary edema. El-Seify et al [13] used a safer method utilizing a 1-ml syringe to perform 0.6-ml nasal dripping of xylometazoline after lubrication with 1 ml lidocaine jelly. We did not prepare the drug but instead used a commercially available xylometazoline spray, which entailed almost no risk of accidental overdose and related complications.
The use of xylometazoline spray instead of nasal packing can achieve similar results with greater ease and prevent complications related to nasal packing with vasoconstrictors. However, patient cooperation is essential to obtain the proper effect of xylometazoline spray. The use of this method can be limited when the patient cannot cooperate with the physician because of various problems including an altered mental status and patient refusal. Moreover, the spray is designed for use while the patient is in an upright position.
The percentage of smooth intubations was high in both groups (XS group: 75%; EP group: 65%). Moreover, the incidence of bleeding was relatively lower in this study than in other previous studies [7812]. Kihara et al. [7] reported that a silicone-based ETT is superior to a polyvinyl chloride (PVC) tube for nasal intubation (the incidence of epistaxis was 32.5% with silicone tubes vs. 80% with PVC tubes). We used the Polar™ Preformed Tracheal Tube, as the ivory-colored PVC tube is much softer than a regular PVC tube. Therefore, there was no need of thermo-softening with warm saline. This may account for some of the differences between the outcomes of the present and previous studies. Various methods including vasoconstrictors have been developed to decrease nasal trauma and bleeding while performing NTI, and aspiration of xylometazoline spray can be a good alternative method for nasal cavity preparation with vasoconstrictors.
This study has some limitations. First, a third control group in which no vasoconstrictor was used was absent; however, this was not considered ethical as the effect of vasoconstrictors is already well known. Thus, it was not considered in the study design. Second, the method of epistaxis assessment was subjective. However, a blinded independent observer assessed the bleeding during intubation and after extubation, and this practice improved the validity of this method.
In conclusion, the two methods of nasal cavity treatment with vasoconstrictors—xylometazoline spray aspiration method and nasal packing with epinephrine jelly—had similar effects, and the incidence of NTI-related complications was not statistically different. On the basis of this result, we propose xylometazoline spray as a good alternative to nasal packing for nasal preparation before NTI.
References
1. Sanuki T, Hirokane M, Kotani J. Epistaxis during nasotracheal intubation: a comparison of nostril sides. J Oral Maxillofac Surg. 2010; 68:618–621. PMID: 19931965.

2. Krebs MJ, Sakai T. Retropharyngeal dissection during nasotracheal intubation: a rare complication and its management. J Clin Anesth. 2008; 20:218–221. PMID: 18502368.

3. Kuo MJ, Reid AP, Smith JE. Unilateral nasal obstruction: an unusual presentation of a complication of nasotracheal intubation. J Laryngol Otol. 1994; 108:991–992. PMID: 7829957.

4. Paul M, Dueck M, Kampe S, Petzke F, Ladra A. Intracranial placement of a nasotracheal tube after transnasal trans-sphenoidal surgery. Br J Anaesth. 2003; 91:601–604. PMID: 14504169.

5. Scamman FL, Babin RW. An unusual complication of nasotracheal intubation. Anesthesiology. 1983; 59:352–353. PMID: 6614546.

6. Arendt KW, Khan K, Curry TB, Tsen LC. Topical vasoconstrictor use for nasal intubation during pregnancy complicated by cardiomyopathy and preeclampsia. Int J Obstet Anesth. 2011; 20:246–249. PMID: 21315577.

7. Kihara S, Komatsuzaki T, Brimacombe JR, Yaguchi Y, Taguchi N, Watanabe S. A silicone-based wire-reinforced tracheal tube with a hemispherical bevel reduces nasal morbidity for nasotracheal intubation. Anesth Analg. 2003; 97:1488–1491. PMID: 14570671.

8. Kim YC, Lee SH, Noh GJ, Cho SY, Yeom JH, Shin WJ. Thermosoftening treatment of the nasotracheal tube before intubation can reduce epistaxis and nasal damage. Anesth Analg. 2000; 91:698–701. PMID: 10960403.

9. Lee JH, Kim CH, Bahk JH, Park KS. The influence of endotracheal tube tip design on nasal trauma during nasotracheal intubation: magill-tip versus murphy-tip. Anesth Analg. 2005; 101:1226–1229. PMID: 16192550.

10. Li YC, Zhang L, Li GH, Li DK, Li C. Establishment of artificial airway with a thermal-softened nasotracheal tube guided by fiberoptic bronchoscope. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007; 19:549–551. PMID: 17767826.
11. Morimoto Y, Sugimura M, Hirose Y, Taki K, Niwa H. Nasotracheal intubation under curve-tipped suction catheter guidance reduces epistaxis. Can J Anaesth. 2006; 53:295–298. PMID: 16527796.

12. Seo KS, Kim JH, Yang SM, Kim HJ, Bahk JH, Yum KW. A new technique to reduce epistaxis and enhance navigability during nasotracheal intubation. Anesth Analg. 2007; 105:1420–1424. PMID: 17959976.

13. El-Seify ZA, Khattab AM, Shaaban AA, Metwalli OS, Hassan HE, Ajjoub LF. Xylometazoline pretreatment reduces nasotracheal intubation-related epistaxis in paediatric dental surgery. Br J Anaesth. 2010; 105:501–505. PMID: 20682569.

14. Haenisch B, Walstab J, Herberhold S, Bootz F, Tschaikin M, Ramseger R. Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam Clin Pharmacol. 2010; 24:729–739. PMID: 20030735.

15. Strandell B, Norgren-Holst E, Tran N, Jakobsen HB, Chen S. OTC use of a topical nasal spray solution containing xylometazoline plus ipratropium in patients with common cold. Int J Clin Pharmacol Ther. 2009; 47:744–751. PMID: 19954713.

16. O'Hanlon J, Harper KW. Epistaxis and nasotracheal intubation--prevention with vasoconstrictor spray. Ir J Med Sci. 1994; 163:58–60. PMID: 7515382.
17. Graf P, Eccles R, Chen S. Efficacy and safety of intranasal xylometazoline and ipratropium in patients with common cold. Expert Opin Pharmacother. 2009; 10:889–908. PMID: 19351236.

18. Son JS, Lee SK. Pulmonary edema following phenylephrine intranasal spray administration during the induction of general anesthesia in a child. Yonsei Med J. 2005; 46:305–308. PMID: 15861508.

Table 1
Patient characteristics
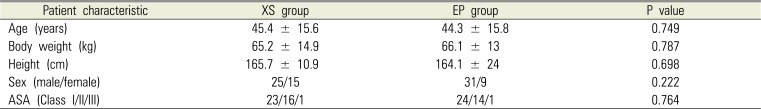
Table 2
Intubation characteristics: navigability
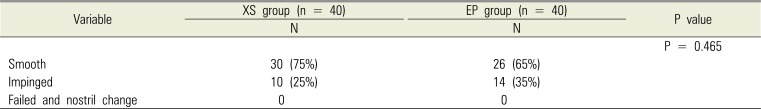
| Variable | XS group (n = 40) | EP group (n = 40) | P value |
|---|---|---|---|
| N | N | ||
| P = 0.465 | |||
| Smooth | 30 (75%) | 26 (65%) | |
| Impinged | 10 (25%) | 14 (35%) | |
| Failed and nostril change | 0 | 0 |
Table 3
Bleeding during intubation procedures and incidence of intubation-related complications




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download