Abstract
Purpose
Metastatic lymph node ratio (MLNR) is known as an important prognostic factor in many solid carcinomas; however, the role of MLNR in papillary thyroid carcinoma (PTC) is unclear. The purpose of this study was to determine whether MLNR has prognostic significance for recurrence in patients with pathological N1a PTC.
Methods
A retrospective analysis was conducted of 1,198 patients with PTC who underwent total thyroidectomy with central neck dissection between 2006 and 2011. Only patients with central lymph node metastasis were included in this study. Patients with lateral neck lymph node metastasis or extrathyroidal involvement were excluded. Finally, this study included 282 patients with N1a patients. MLNR was defined as the number of metastatic lymph nodes divided by the number of removed lymph nodes.
Results
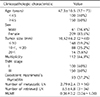
Median age was 47.3 years (17~73 years). There were 209 female patients and 41 male patients, respectively. Median follow-up period was 53 months (36~114 months). Median value of MLNR was 0.36 (0.04~1.000). Of 250 patients, 20 patients (8.0%) developed recurrent disease. MLNR independently predicted PTC recurrence (odds ratio [OR], 6.385; 95% confidence interval [CI], 2.523-16.158; P < 0.001). In receiver operating characteristic curve analysis, 0.47 was significantly meaningful for recurrence when three or more lymph nodes were collected.
Figures and Tables
Fig. 2
Recurrence-free survival curves according to metastatic lymph node ration (MLNR) in patients with pN1a papillary thyroid carcinoma. There was a significant difference between patients with MLNR 0.47 and ≥ 0.47 (P<0.001).

Fig. 3
In tumor size ≤1 cm group and 1 cm <tumor size ≤2 cm group, there was a significant difference in recurrencefree survival in patients group according to metastatic lymph node ratio (MLNR) (P=0.044; P=0.004; respectively) (A, B). However, there was no difference in tumor size >2 cm group (P=0.386) (C).

References
1. Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002; 131:249–256.

2. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990; 71:414–424.

3. Tubiana M, Schlumberger M, Rougier P, Laplanche A, Benhamou E, Gardet P, et al. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer. 1985; 55:794–804.

4. Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005; 71:731–734.

5. Reddy RM, Grigsby PW, Moley JF, Hall BL. Lymph node metastases in differentiated thyroid cancer under 2 cm. Surgery. 2006; 140:1050–1054. discussion 1054-5.

6. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004; 135:139–148.

7. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.

8. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.

9. Lee YS, Lim YS, Lee JC, Wang SG, Kim IJ, Lee BJ. Clinical implication of the number of central lymph node metastasis in papillary thyroid carcinoma: preliminary report. World J Surg. 2010; 34:2558–2563.

10. Jeon MJ, Yoon JH, Han JM, Yim JH, Hong SJ, Song DE, et al. The prognostic value of the metastatic lymph node ratio and maximal metastatic tumor size in pathological N1a papillary thyroid carcinoma. Eur J Endocrinol. 2013; 168:219–225.

11. Lee SR, Kim HO, Son BH, Shin JH, Yoo CH. Prognostic significance of the metastatic lymph node ratio in patients with gastric cancer. World J Surg. 2012; 36:1096–1101.

12. Tausch C, Taucher S, Dubsky P, Seifert M, Reitsamer R, Kwasny W, et al. Prognostic value of number of removed lymph nodes, number of involved lymph nodes, and lymph node ratio in 7502 breast cancer patients enrolled onto trials of the Austrian Breast and Colorectal Cancer Study Group (ABCSG). Ann Surg Oncol. 2012; 19:1808–1817.

13. Ren JQ, Liu JW, Chen ZT, Liu SJ, Huang SJ, Huang Y, et al. Prognostic value of the lymph node ratio in stage III colorectal cancer. Chin J Cancer. 2012; 31:241–247.

14. Noguchi M, Kumaki T, Taniya T, Miyazaki I. Bilateral cervical lymph node metastases in well-differentiated thyroid cancer. Arch Surg. 1990; 125:804–806.

15. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012; 22:1144–1152.

16. Ryu IS, Song CI, Choi SH, Roh JL, Nam SY, Kim SY. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol. 2014; 21:277–283.

17. Vas Nunes JH, Clark JR, Gao K, Chua E, Campbell P, Niles N, et al. Prognostic implications of lymph node yield and lymph node ratio in papillary thyroid carcinoma. Thyroid. 2013; 23:811–816.

18. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:418–428.

19. Monzen S, Mariya Y, Wojcik A, Kawamura C, Nakamura A, Chiba M, et al. Predictive factors of cytotoxic damage in radioactive iodine treatment of differentiated thyroid cancer patients. Mol Clin Oncol. 2015; 3:692–698.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download