Abstract
Background
Glioblastoma multiforme (GBM) is a highly malignant brain tumor with a worst prognosis of less than one year despite advance treatment facilities. Among various signaling pathway genes displaying genetic modifications, aberrant expression of Notch pathway genes is frequent in GBM offering novel therapeutic targets. Herbal extracts having anticancer properties are used in adjuvant therapy that is safe and affordable as compared to chemotherapeutics. Bacopa monnieri has been used for the development of brain cells because of its neuroprotective properties. Its anticancer properties have shown to be promising in cancer treatment.
Methods
The anticancer properties of Bacoside A, an active and abundant component of Bacopa monnieri was assessed on U-87 MG cell line and its effects on expression of Notch pathway genes were studied. Cell cycle arrest and apoptosis were studied using flow cytometry. Expression of Notch pathway genes comprising of Notch receptors (notch1, notch2, notch3 and notch4), ligands (jagged1 and jagged2), a component of gamma-secretase complex (APH1A) and downstream target (HES1) were evaluated by quantitative real-time PCR.
Results
Bacoside A exhibited considerable cytotoxicity on U-87 MG cells inducing cell cycle arrest and apoptosis. Cell cycle analysis revealed a significant arrest of 39.21% cells in sub-G0 phase at 80 µg/mL concentration, increasing to 53.21% at a higher concentration of 100 µg/mL. The fraction of early apoptotic cells in control was low (3.48%) that increased substantially to 31.36% and 41.11% after 80 µg/mL and 100 µg/mL of Bacoside A treatment respectively. Additionally, the expression of notch1 gene decreased after exposure to Bacoside A with a fold change of 0.05, whereas HES1 gene expression was increased by 25 fold.
Glioblastoma multiforme (GBM), a grade IV glioma, is the most common and most aggressive primary brain tumor [1]. Additionally, GBM is highly malignant having poor prognosis, with a median survival of 12 to 15 months [2]. The standard therapy for GBM is surgery followed by radiation and chemotherapy. The effect of currently used chemotherapeutic drug Temozolomide is not satisfactory since there is no significant improvement in patient survival. Furthermore, a number of genetic alterations including several cell signaling pathway genes are involved in GBM pathogenesis. One such alteration that occurs frequently in GBM is aberrant expression of Notch signaling pathway genes. The Notch signaling pathway plays a critical role in maintenance and self-renewal of neural stem cells [3]. Since Notch pathway is involved in maintaining cells in an undifferentiated state, dysregulated Notch genes have been associated with a growing list of cancers including T-cell acute lymphoblastic leukemia, Hodgkin lymphoma, multiple myeloma, glioma, cervical, pancreatic, lung, breast cancer, hepatocellular carcinoma, etc. [45]. In an attempt to better understand GBM, several studies have examined genetic alterations in Notch signaling pathway [678]. Also, Notch inhibition has been shown to result in decreased proliferation and self-renewal of GBM cells in several instances [91011]. Notch signaling pathway has thus proved to be a novel therapeutic target in the treatment of GBM.
Numerous studies have documented various herbal products as novel anticancer drugs for efficient cancer treatment. Currently used chemotherapeutic agents are systemic and cause harmful side effects. Compared to highly toxic and expensive chemotherapeutic agents, herbal products have enabled the development of safer, nontoxic and affordable treatment for cancer. Bacoside A is the most studied constituent of Bacopa monnieri, locally known as Brahmi. Brahmi has been used for centuries in Ayurveda as a rejuvenating herb for nerve and brain cells development because of its neuroprotective properties. Bacopa monnieri has been found to have antioxidant properties which is used to treat oxidative stress caused due to aging and cigarette smoke [1213]. It is observed to inhibit intraneuronal lipofuscin accumulation and necrotic alteration caused due to neurotoxicity in the hippocampus [14]. Its ability to cross the blood brain barrier has been exploited in the treatment of Alzheimer's and Parkinson's disease, attention deficit disorder, etc. Additionally, Bacopa monnieri as a crude plant extract or as individual constituents have been used in the treatment of cancer. However, the antitumor property of Brahmi has not been studied extensively in recent times. Various in vitro and in vivo studies have shown cytotoxic and antiproliferative properties of Bacopa monnieri whole plant extract [1516]. Furthermore, research have suggested that the anticancer effect of Bacopa monnieri extracts is possibly due to inhibition of DNA replication, DNA fragmentation and activation of apoptotic pathway [1718]. Collectively, these data suggest that Brahmi emerges as a promising therapy and further research needs to be done to check the antitumor property of the herb in treating GBM.
In the present study, we explored the antitumor properties of a bioactive phytochemical Bacoside A in human GBM cell line U-87 MG and its effects on the expression of Notch signaling pathway genes. This could enhance our understanding of the role of Notch genes in GBM and present a specific molecular target for developing a much needed herbal therapy to treat GBM.
Human Glioblastoma cell line U-87 MG (ATCC, Manassas, VA, USA) was cultured in DMEM (Sigma Aldrich, St. Louis, MO, USA) supplemented with 10% FBS (Invitrogen, Camarillo, CA, USA). The cells were cultured in T25 flasks and grown in a humidified 5% CO2 incubator at 37℃. The cells were trypsinized and sub cultured on reaching 70–80% confluence. Bacoside A (Natural Remedies, Bangalore, India) was dissolved in HPLC grade methanol (Fisher Scientific, Waltham, MA, USA) to obtain a stock solution of 3.2 mg/mL concentration by making up the volume with DMEM. After preliminary dose response studies (data not shown), working solutions were prepared from the stock at concentrations of 320, 160, 80, 40, 20, 10, and 5 µg/mL with DMEM.
The cells were seeded in a 96-well plate with 50,000 cells per well in medium and incubated for 24 h at 37℃, 5% CO2 and 95% air atmosphere. After 24 h incubation, cells were treated with different concentrations of Bacoside A (320, 160, 80, 40, 20, 10 and 5 µg/mL), each in triplicate for another 24 h. The cells were then harvested and washed with 1× PBS (HiMedia, Mumbai, India). 100 µL of MTT (0.5 mg/mL) (Sigma Aldrich) was added to each well and incubated for 3.5 h at 37℃ before 100 µL of 100% DMSO (HiMedia) was added. The absorbance was measured on a microplate reader (Tecan, Männedorf, Switzerland) at a wavelength of 490 nm. The solvent methanol treated cells served as control. The final concentration of methanol in culture medium did not exceed 1% and was proved not to affect the experiment. Percentage inhibition was expressed using the following formula:
IC50 value was determined by plotting % inhibition on y-axis against concentration of Bacoside A on x-axis which gives the concentration of drug required for 50% inhibition. IC50 values were derived from a non-linear regression model (curvefit), computed using GraphPad Prism (La Jolla, CA, USA).
Cell cycle analysis was performed by flow cytometry using propidium iodide (PI) based measurements of the DNA content in the cells. Briefly, U-87 MG cells were treated with 80 µg/mL and 100 µg/mL Bacoside A for 24 h. Treated and untreated cells were harvested and washed twice with 1× PBS (4,000 rpm, 10 min, 25℃). Thereafter, cells were fixed with 70% ice cold ethanol and stored at 4℃ overnight. The cells were then washed twice with 1× PBS (4,000 rpm, 10 min, 25℃). Later, cells were stained with 500 µL of PI (0.05 mg/mL in PBS containing 0.05 mg/mL RNase A) for 15 min at room temperature in dark. Cells were analyzed for DNA content on a FACSCalibur™ flow cytometer using Cell Quest acquisition and analysis software (Becton Dickinson, Heidelberg, Germany).
U-87 MG cells were incubated with 80 µg/mL and 100 µg/ mL Bacoside A for 24 h before the cells were harvested. The treated and untreated cells were washed twice in 1× PBS (4,000 rpm, 10 min, 25℃), and the cell pellet obtained was resuspended in Annexin V binding buffer at room temperature to a concentration of 1×106 cells/mL. To the resuspended solution, 5 µL of Annexin V-FITC and 5 µL of PI were added. The resulting mixtures were gently mixed and incubated for 15 min at room temperature in dark. An additional 400 µL of binding buffer was added to each tube and flow cytometric evaluation was conducted immediately on a FACSCalibur™ flow cytometer using Cell Quest acquisition and analysis software (Becton Dickinson).
The Annexin V-FITC/PI staining procedure of the treated and untreated cells was performed as above. After staining, 20 µL of sample was mounted onto a glass slide with a cover slip and observed under 40× objective lens of fluorescence microscope (Lawrence & Mayo, Mumbai, India). Images of cells were obtained with a digital camera (DSC-W830, Sony, Tokyo, Japan) connected to the microscope.
U-87 MG cells were cultured for 24 h before treating with 80 µg/mL and 100 µg/mL of Bacoside A and grown at 37℃ in a humidified 5% CO2 incubator for 24 h. At the end of treatment, both floating cells and adherent cells were harvested. Genomic DNA was isolated from treated and untreated cells by TRIzol (Invitrogen) method as recommended by the manufacturer. DNA samples were mixed with loading dye (Bangalore GeNei, Bangalore, India) and resolved in 1% agarose gel (Lonza, Basel, Switzerland) containing 0.5 µg/mL ethidium bromide (Sigma Aldrich). Electrophoresis was carried out at 75 V for 60 min using 1× TAE buffer (0.04 M Tris acetate, 0.001 M EDTA, pH 8.2). After electrophoresis, DNA in the gel was visualized and photographed in a gel documentation system (UVItec, Cambridge, UK).
U-87 MG cells were treated with 80 µg/mL of Bacoside A corresponding to their IC50 value for 24 h. Treated and untreated cells were harvested and total RNA was extracted by TRIzol (Invitrogen) method as recommended by the manufacturer. RNA samples extracted were quantified using NanoDrop 1,000 spectrophotometer (Thermo Scientific, DE, USA). 1 µg of RNA was reverse transcribed to cDNA using high-capacity cDNA reverse transcription kit (Applied Biosystems, CA, USA) as per manufacturer's protocol.
The relative quantitation of expression levels of eight Notch pathway genes (notch1, notch2, notch3, notch4, jagged1, jagged2, APH1A and HES1) was carried out on Applied Biosystems StepOnePlus™ Instrument (CA, USA). Amplification was performed using SYBR Green master mix (Fermentas, MA, USA), 150 nM forward and reverse primer (Sigma Aldrich) and 20 ng of cDNA template. Amplification was carried out with an initial denaturation step at 95℃ for 10 min followed by 45 cycles at 95℃ for 15 s, 57℃ for 30 s and 72℃ for 30 s in a total of 12.5 µL reaction volume. All reactions were run in triplicates and the mean was used for further calculations. TATA binding protein (TBP), a housekeeping gene was chosen as an appropriate internal control after validating four conventionally used housekeeping genes (GAPDH, TBP, B2M and RPL13A) in gene expression studies (data not shown). Untreated cells were used as calibrator. Relative quantification was done by 2−ΔΔCt method, the data are presented as fold change in gene expression normalized to an endogenous reference gene and relative to the calibrator, and is given by:
Statistical significance was assessed by calculating probability values through Student's t test using GraphPad. p values less than 0.05 were considered as significant.
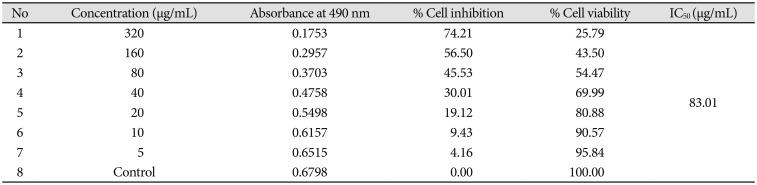
Cytotoxic effect of Bacoside A was evaluated on U-87 MG cells using the MTT colorimetric assay. Results indicated that Bacoside A possessed dose-dependent cytotoxic effects against human GBM cells (Fig. 1, Table 1). It is evident from the results in the Table 1 that the cell viability was not significantly affected at concentrations up to 20 µg/mL of Bacoside A, which retained 80.88% of viable cells. However, 74.21% inhibition was observed at concentration 320 µg/mL. Thus, to exclude the cytotoxicity of excess Bacoside A, IC50 value was determined and found to be 83.01 µg/mL against U-87 MG cell line.
In order to evaluate the effects of Bacoside A on cell cycle, U-87 MG cells were treated with the drug at 80 µg/mL and 100 µg/mL concentration for 24 h. DNA content in sub-G0 phase, G0/G1 phase, S phase, and G2/M phase were determined at the end of the treatment by flow cytometry. Fig. 2 and 3 shows the effects of Bacoside A on sub-G0 phase, G0/G1 phase, S phase and G2/M phase in U-87 MG cells after 24 h treatment. Untreated cells showed a normal cell cycle with hardly any sub-G0 DNA (Fig. 2A, Fig. 3). Treatment with Bacoside A caused a significant increase in sub-G0 phase, indicative of apoptosis. This increase in sub-G0 cell population was accompanied with a decrease in the population of cells in G0/G1, S and G2/M phase. The treatment at 80 µg/mL concentration caused an arrest of 39.21% cells in sub-G0 phase, further increasing to 53.21% at the higher concentration of 100 µg/mL compared to untreated cells (0.37%) (p=0.022) (Fig. 2B and C, Fig. 3). Thus, these results revealed that an increase of Bacoside A concentration led to increased apoptosis in human GBM cell line.
By staining Bacoside A treated U-87 MG cells with Annexin V-FITC and PI, flow cytometry was carried out to determine the percentage of apoptotic and necrotic cells after 24 h treatment. The percentage of viable U-87 MG cells (AnV−/PI−) after 80 µg/mL and 100 µg/mL of Bacoside A treatment decreased to 67.77% and 58.56% respectively when compared with the untreated control (93.87%) (p=0.021). Furthermore, the percentage of early apoptotic cells (AnV+/PI−) increased from 3.48% in untreated control cells to 31.36% and 41.11% in 80 µg/mL and 100 µg/mL of Bacoside A treated cells respectively (p=0.021) (Fig. 4). Thus, Bacoside A induces early apoptosis in human GBM cell line in a dose dependent manner.
U-87 MG cells treated with Bacoside A were stained with Annexin V-FITC/PI and observed under a fluorescence microscope. Bacoside A treated cells when stained with PI displayed red fluorescence due to fragmented chromatin and green fluorescence on the cell membrane when stained with Annexin V-FITC. Different labelling patterns enabled identification of different cell populations. Early apoptotic cells showed affinity towards Annexin V-FITC alone and were devoid of PI (Fig. 5A). Late apoptotic/necrotic cells showed affinity for both Annexin V-FITC and PI displaying red nucleus and a halo of green on the cell surface (Fig. 5B). Dead cells showed affinity towards PI alone indicating completely degraded cell membrane (Fig. 5C).
DNA from Bacoside A treated U-87 MG cells showed DNA ladder formation (Fig. 6) which is a hallmark property of apoptosis further confirming the induction of cell death by the drug. However, DNA from the control cells was not degraded. Together, these results could indicate that Bacoside A induces apoptosis in human GBM cells in vitro.
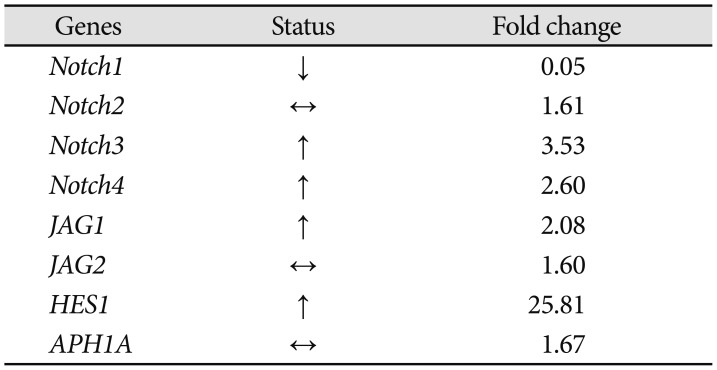
To examine if Bacoside A induced cell death via Notch signaling pathway, we studied expression of eight Notch pathway genes by real-time PCR to determine whether Notch pathway genes were modulated by Bacoside A. Bacoside A did not affect the expression levels of notch2, notch3, notch4, JAG1, JAG2 and APH1A genes as compared to the untreated control cells (Fig. 7). However, the expression of Notch receptor notch1 was decreased significantly with a fold change of 0.05 (p=0.005) and the expression of Notch downstream target HES1 was increased by 25 fold after 80 µg/mL of Bacoside A treatment for 24 h (p=0.017) (Table 2, Fig. 7). These results provide added data for Bacoside A induced apoptosis via the Notch signaling pathway in U-87 MG cells.
GBM is a complex disease which is extremely refractory to therapy, in part because of poor understanding of several genetic alterations involved in its pathogenesis. Most of the patients who succumb to GBM within one year despite advance treatment highlight the need for better therapies acting at the genetic level. In recent years, herbal therapy has gradually emerged as a novel cancer treatment having a huge advantage over conventional chemotherapeutics. The common features of inducing cell cycle arrest and apoptosis by chemotherapic agents in cancer cells are the focus of this study. We investigated the anticancer property of Bacoside A which is the most studied component of Bacopa monnieri, against human GBM cell line U-87 MG. Untreated cells were used as experimental control in this study. Bacoside A has gained significant attention in recent years as a lead for the development of anticancer therapeutics [192021]. It is a blend of bacoside A3, bacopacide II, bacopasaponin C, and a jujubogenin isomer of bacosaponin C [22]. It is however found to be safe and nontoxic on normal cells making it a suitable therapeutic lead [232425]. The aim of this work was to determine the effects of Bacoside A on cell cycle arrest and apoptosis with associated impact on expression of Notch pathway genes in human GBM cell line U-87 MG.
Evaluation of cytotoxicity revealed that Bacoside A showed dose-dependent cytotoxicity against U-87 MG cells. The IC50 value was found to be less than 100 µg/mL signifying potent cytotoxic effects in GBM cells. We investigated the cell cycle specific effects of Bacoside A against human GBM cells by PI based cell cycle analysis using flow cytometry. Cancer cell cycle specific drugs cause cell death by blocking cell cycle at specific cell cycle checkpoints (sub-G0, G0/G1 phase, S phase and G2/M phase) [26]. These drugs could induce apoptosis (sub-G0 arrest) or inhibit cell proliferation (G0/G1 phase arrest), or DNA replication (S phase arrest) or mitosis (G2/M phase arrest) [27]. The flow cytometry result showed an arrest of 39.21% cells in sub-G0 phase at 80 µg/mL concentration, further increasing to 53.21% at the higher concentration of 100 µg/mL after 24 h. It is possible that the toxic effect of high doses of Bacoside A may inhibit the enzymes associated with cell cycle. The significant arrest of cells at sub-G0 phase is indicative of apoptosis thus suggesting that Bacoside A act by cell cycle-specific mechanism inducing apoptosis in GBM cells.
Most anti-cancer drugs eliminate rapidly proliferating cancerous cells, by inducing apoptosis [28]. Apoptosis is characterized by disintegration of plasma membrane, enzymatic cleavage of the DNA into fragments, and formation of apoptotic bodies [29]. We investigated the induction of apoptosis in GBM cells upon treatment with Bacoside A. Annexin V-FITC/PI assay by flow cytometry revealed that Bacoside A induced early apoptosis, but not necrosis, in GBM cells. Consistent with the MTT assay, the Annexin V-FITC/PI staining and DNA fragmentation studies demonstrated significant growth inhibitory effect of Bacoside A on GBM cells. DNA fragmentation which is a hallmark property of apoptosis further confirmed apoptosis in GBM cells. Furthermore, the morphology of the cells changed and the number of viable cells decreased under microscopic examinations.
Treatment of GBM cells with Bacoside A decreased notch1 expression and increased the levels of HES1 expression. Numerous studies have documented a direct correlation between high levels of notch1 expression and GBM pathogenesis [830]. Induced overexpression of HES1
in vitro and in vivo led to growth arrest and apoptosis in acute myeloid leukaemia and hepatocellular carcinoma supporting the tumour suppressor role of HES1 in these cancers [313233]. Furthermore, gastric and prostate cancer cells with lower levels of notch1 were prone to apoptosis and decreased cell proliferation [3435]. Pretreatment of glioma cells with Notch1 siRNA prolonged survival in a murine orthotopic brain tumor model [8]. Furthermore, inhibition of Notch signaling through knockdown of notch receptors in GBM resulted in significant reduction in cell growth in vitro and in vivo [9]. Notch blockade in GBM derived neurosphere cultures by gamma secretase inhibitors reduced neurosphere growth and stem cell markers [36]. Additionally, there are studies showing increased expression of HES1 due to interference with notch1 expression inhibiting glioma cancer cells growth by inducing cell autophagy [37] and overexpression of HES1 inhibiting Notch1 signaling which in turn decreased tumor-initiating subpopulation in breast cancer [38]. Thus, these studies evidently display the oncogenic property of notch1 gene and tumor suppressor role of HES1 gene. Therefore, we report here that Bacoside A induced apoptosis in GBM cells in vitro may be dependent on Notch signaling pathway. The decreased levels of notch1 and increased levels of HES1 may, in part, account for the high susceptibility of GBM cells to cell cycle arrest and apoptosis in response to Bacoside A.
Taken together, alterations in the expression of few Notch genes, DNA fragmentation, cell cycle arrest and apoptosis indicate that, Bacoside A may induce cell death in GBM. Both the levels of Notch genes and morphological changes in GBM cells after treatment with Bacoside A suggest a Notch-dependent cytotoxicity. This is also proved by the flow cytometric analysis showing cell cycle arrest and early apoptosis. Thus, Bacoside A via a novel mechanism of regulating Notch gene expression may provide a promising lead for GBM therapy. These findings thus provide new insights into the possible molecular mechanism of the cytotoxic activity of Bacoside A on GBM cells.
However, there are few limitations that need to be reported which could help in shaping the future research agenda. The effects of Bacoside A on the basic components of cell cycle regulation including cyclin-dependent kinases and their regulators, and apoptosis including caspases should be studied. To obtain the first mechanistic insights into the scenarios of Bacoside A induced cell death in vitro, a cell line that is well-established and most widely used in GBM studies was chosen. With these preliminary data, the efficacy of the compound should be subsequently extended to observe the effects on other GBM cell lines particularly IDH mutant and wild-type.
Acknowledgments
This study was funded by the Department of Biotechnology, New Delhi, India (grant number 6242-P20/RGCB/PMD/DBT/NRWR/2015). We thank Department of Neurovirology, NIMHANS, Bangalore, India, for the technical support. We thank Skanda Life Sciences, Bangalore, India for providing the flow cytometry and cell culture facility to carry out the work.
References
1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014; 16(Suppl 4):iv1–iv63. PMID: 25304271.

3. Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010; 30:3489–3498. PMID: 20203209.

4. Axelson H. Notch signaling and cancer: emerging complexity. Semin Cancer Biol. 2004; 14:317–319. PMID: 15288256.

5. Hansson EM, Lendahl U, Chapman G. Notch signaling in development and disease. Semin Cancer Biol. 2004; 14:320–328. PMID: 15288257.

6. Dell'albani P, Rodolico M, Pellitteri R, et al. Differential patterns of NOTCH1-4 receptor expression are markers of glioma cell differentiation. Neuro Oncol. 2014; 16:204–216. PMID: 24305720.
7. Narayanappa R, Rout P, Aithal MG, Chand AK. Aberrant expression of Notch1, HES1, and DTX1 genes in glioblastoma formalin-fixed paraffin-embedded tissues. Tumour Biol. 2016; 37:6935–6942. PMID: 26662803.

8. Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005; 65:2353–2363. PMID: 15781650.

9. Chen J, Kesari S, Rooney C, et al. Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 2010; 1:822–835. PMID: 21127729.

10. Hovinga KE, Shimizu F, Wang R, et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010; 28:1019–1029. PMID: 20506127.

11. Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006; 444:1083–1087. PMID: 17183323.

12. Anbarasi K, Vani G, Balakrishna K, Devi CS. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006; 78:1378–1384. PMID: 16226278.

13. Simpson T, Pase M, Stough C. Bacopa monnieri as an antioxidant therapy to reduce oxidative stress in the aging brain. Evid Based Complement Alternat Med. 2015; 2015:615384. PMID: 26413126.
14. Jyoti A, Sharma D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology. 2006; 27:451–457. PMID: 16500707.

15. Kumar EP, Elshurafa AA, Elango K, Subburaju T, Suresh B. Cytotoxic and anti -tumour properties of ethanolic extract of bacopa monnieri (L) penn. Anc Sci Life. 1998; 17:228–234. PMID: 22556847.
16. Peng L, Zhou Y, Kong de Y, Zhang WD. Antitumor activities of dammarane triterpene saponins from Bacopa monniera. Phytother Res. 2010; 24:864–868. PMID: 19960417.

17. Elangovan V, Govindasamy S, Ramamoorthy N, Balasubramanian K. In vitro studies on the anticancer activity of Bacopa monnieri. Fitoterapia. 1995; 66:211–215.
18. Kalyani MI, Lingaraju SM, Salimath BP. A pro-apoptotic 15-kDa protein from Bacopa monnieri activates caspase-3 and downregulates Bcl-2 gene expression in mouse mammary carcinoma cells. J Nat Med. 2013; 67:123–136. PMID: 22467255.

19. Janani P, Sivakumari K, Geetha A, Ravisankar B, Parthasarathy C. Chemopreventive effect of bacoside A on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats. J Cancer Res Clin Oncol. 2010; 136:759–770. PMID: 19916024.

20. Janani P, Sivakumari K, Geetha A, Yuvaraj S, Parthasarathy C. Bacoside A downregulates matrix metalloproteinases 2 and 9 in DEN-induced hepatocellular carcinoma. Cell Biochem Funct. 2010; 28:164–169. PMID: 20084675.

21. Prakash NS, Sundaram R, Mitra SK. In vitro and in vivo anticancer activity of Bacoside A from whole plant of Bacopa monnieri (Linn). Am J Pharmacol Toxicol. 2011; 6:11–19.
22. Deepak M, Sangli GK, Arun PC, Amit A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem Anal. 2005; 16:24–29. PMID: 15688952.
23. John S, Sivakumar KC, Mishra R. Bacoside A induces tumor cell death in human glioblastoma cell lines through catastrophic macropinocytosis. Front Mol Neurosci. 2017; 10:171. PMID: 28663722.

24. Rauf K, Subhan F, Al-Othman AM, Khan I, Zarrelli A, Shah MR. Preclinical profile of bacopasides from Bacopa monnieri (BM) as an emerging class of therapeutics for management of chronic pains. Curr Med Chem. 2013; 20:1028–1037. PMID: 23210787.

25. Singh HK. Neuropsychopharmacological effects of the ayurvedic nootropic Bacopa monnieri Linn. Indian J Pharmacol. 1997; 29:S359–S365.
26. Stewart ZA, Westfall MD, Pietenpol JA. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol Sci. 2003; 24:139–145. PMID: 12628359.

27. Nunez R. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol. 2001; 3:67–70. PMID: 11488413.
28. Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000; 256:42–49. PMID: 10739650.

29. Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009; 9:501–507. PMID: 19550425.

30. Zhang XP, Zheng G, Zou L, et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol Cell Biochem. 2008; 307:101–108. PMID: 17849174.

31. Kannan S, Sutphin RM, Hall MG, et al. Notch activation inhibits AML growth and survival: a potential therapeutic approach. J Exp Med. 2013; 210:321–337. PMID: 23359069.

32. Tian C, Yu Y, Jia Y, Zhu L, Zhang Y. HES1 activation suppresses proliferation of leukemia cells in acute myeloid leukemia. Ann Hematol. 2015; 94:1477–1483. PMID: 26092281.

33. Viatour P, Ehmer U, Saddic LA, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011; 208:1963–1976. PMID: 21875955.

34. Wei G, Chang Y, Zheng J, et al. Notch1 silencing inhibits proliferation and invasion in SGC 7901 gastric cancer cells. Mol Med Rep. 2014; 9:1153–1158. PMID: 24469571.
35. Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ, He LY. Silencing Notch-1 induces apoptosis and increases the chemosensitivity of prostate cancer cells to docetaxel through Bcl-2 and Bax. Oncol Lett. 2012; 3:879–884. PMID: 22741011.

36. Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010; 28:5–16. PMID: 19904829.
37. Yao J, Zheng K, Li C, Liu H, Shan X. Interference of Notch1 inhibits the growth of glioma cancer cells by inducing cell autophagy and downregulation of Notch1-Hes-1 signaling pathway. Med Oncol. 2015; 32:610. PMID: 25920606.

38. So JY, Wahler J, Das Gupta S, et al. HES1-mediated inhibition of Notch1 signaling by a Gemini vitamin D analog leads to decreased CD44(+)/CD24(−/low) tumor-initiating subpopulation in basal-like breast cancer. J Steroid Biochem Mol Biol. 2015; 148:111–121. PMID: 25541438.

Fig. 1
Effect of Bacoside A on human glioblastoma multiforme cell line U-87 MG as determined by MTT assay.
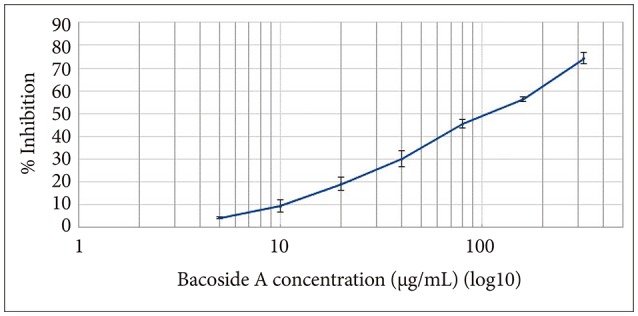
Fig. 2
Cell cycle profiles of untreated (A) and treated U-87 MG cells with 80 µg/mL (B) and 100 µg/mL (C) of Bacoside A respectively for 24 h as determined by flow cytometry.
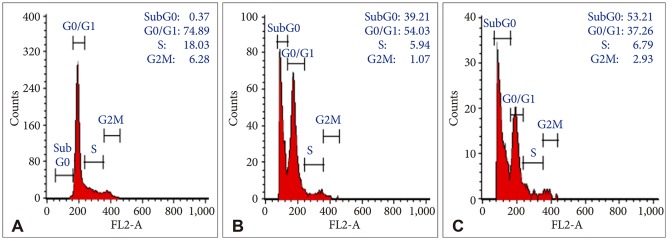
Fig. 3
Bar graph obtained from cell cycle analysis of control, 80 µg/mL and 100 µg/mL of Bacoside A respectively showing the percentage of cells in different phases of cell cycle (G2/M, S, G0/G1 and Sub-G0).
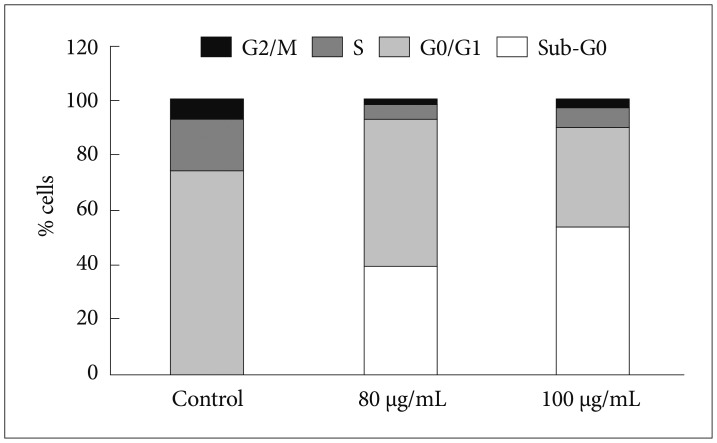
Fig. 4
Apoptosis in untreated (A) and treated U-87 MG cells with 80 µg/mL (B) and 100 µg/mL (C) of Bacoside A respectively for 24 h as determined by flow cytometry.
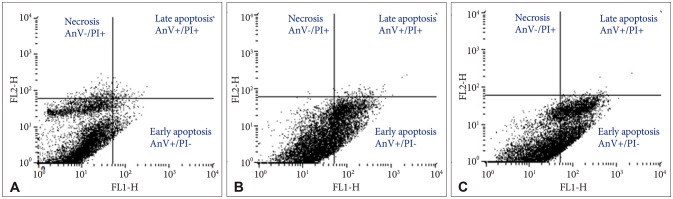
Fig. 5
U-87 MG cells treated with Bacoside A stained with Annexin V-FITC/PI displaying early apoptotic cells (A), late apoptotic/necrotic cells (B) and dead cells (C) as observed under a fluorescent microscope. Scale bar, 50 µm.
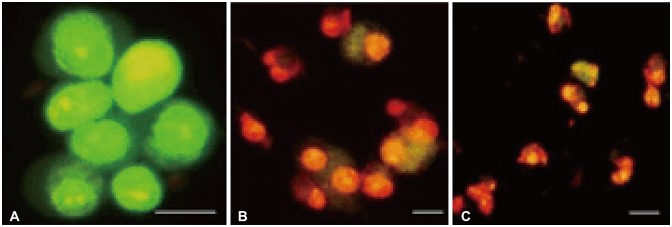
Fig. 6
Bacoside A induced DNA fragmentation in U-87 MG cells analysed by agarose gel electrophoresis.
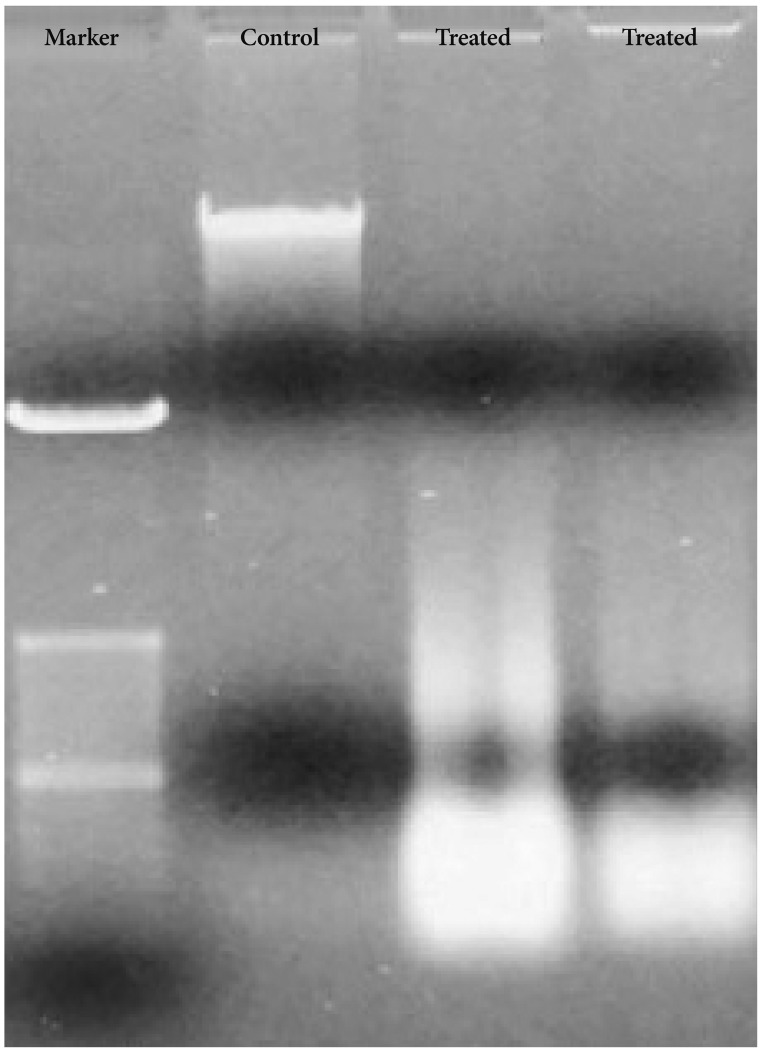
Fig. 7
Scatter plots of differentially expressed Notch pathway genes in Bacoside A treated U-87 MG cells (open circles) compared to untreated cells (filled circles) at 24 h incubation in terms of fold change derived from real-time PCR analysis.
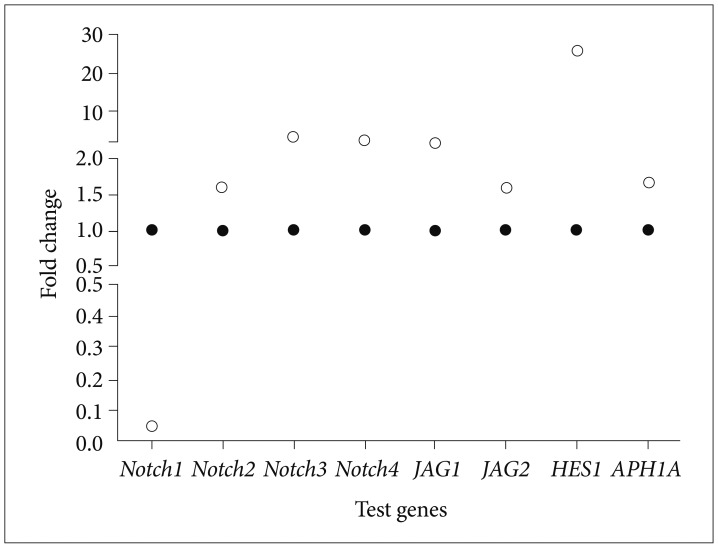
Table 1
Cytotoxicity of bacoside A on human glioblastoma multiforme cell line U-87 MG as determined by MTT assay




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download