Abstract
Gliosarcoma (GS), known as variant of glioblastoma multiforme, is aggressive and very rare primary central nervous system malignant neoplasm. They are usually located in the supratentorial area with possible direct dural invasion or only reactive dural thickening. However, in this case, GS was located in lateral side of left posterior cranial fossa. A 78-year-old man was admitted to our hospital with 3 month history of continuous dizziness and gait disturbance without past medical history. A gadolinium-enhanced MRI demonstrated 5.6×4.8×3.2 cm sized mass lesion in left posterior cranial fossa, heterogeneously enhanced. The patient underwent left retrosigmoid craniotomy with navigation system. The tumor was combined with 2 components, whitish firm mass and gray colored soft & suckable mass. On pathologic report, the final diagnosis was GS of WHO grade IV. In spite of successful gross total resection of tumor, we were no longer able to treat because of the patient's rejection of adjuvant treatment. The patient survived for nine months without receiving any special treatment from the hospital.
Gliosarcoma (GS), known as variant of glioblastoma multiforme (GBM), is aggressive and very rare primary central nervous system (CNS) malignant neoplasm [1]. They are usually located in the supratentorial area with possible direct dural invasion or only reactive dural thickening [2]. In our case, GS was located in lateral side of left posterior cranial fossa. So, we report on the successful surgical resection of posterior cranial fossa GS and review of literature. The Institutional Review Board (IRB) approval was accomplished (SCHCA 2018-07-028).
A 78-year-old man was admitted to our hospital with 3 months history of continuous dizziness and gait disturbance. The patient underwent gastrectomy for gastric cancer 15 years ago. A contrast-enhanced CT (WCT-1000-140 Brilliance iCT, Philips, USA) scan showed huge densely enhancing solid mass with peripheral cystic component in the left posterior fossa, attached the left tentorium, with surrounding brain edema. A gadolinium-enhanced MRI (3.0T Archieva, Philips, Best, the Netherlands) demonstrated 5.6×4.8×3.2 cm sized mass lesion in left posterior cranial fossa, heterogeneously enhanced. Diffusion-weighted imaging showed high signal intensity for solid mass surrounded by low signal intensity of cystic component (Fig. 1). Dural tail sign was noted, but it was suspicious for infiltrative appearance into left cerebellum. Intratumoral hemorrhage was also noted. So, high grade meningioma or dural metastasis was suspected and surgery was planned.
The patient underwent left retrosigmoid craniotomy with navigation system. The tumor was combined with 2 components, whitish firm mass and gray colored soft & suckable mass. The mass was well-demarcated and attached to the dura with severe adhesion. The CN VII & VIII nerve complex and lower cranial nerve was preserved well. Additionally, tumorlike component at the base also removed with minimal lobectomy. Duroplasty with artificial dura was done. The procedure was well tolerated and the patient recovered without complication. The postoperative MRI showed no definite evidence of remaining enhancing tumor at the operation site. We examined the function of swallowing and gag reflex, and the results showed complete recovery of swallowing and gag reflex at the videofluoroscopic swallowing study.
On pathologic report, the final diagnosis was GS of WHO grade IV. Gliofibrillary cytoplasmic processes was positive in glial component, but negative in sarcomatous component. And S-100 protein and P53 was positive in both component. Immuno-histologic staining showed Ki-67 labeling index as high as 20% (Fig. 2).
After surgical treatment, the patient was discharged without any surgical complication. Before pathological confirmation, abdomen & pelvic CT with contrast was done for systemic evaluation and there was no abnormal finding. We recommended concurrent chemoradiotherapy as a standard protocol of GBM, but the patient rejected all treatment to get a home remedies. The patient survived for nine months without receiving any special treatment from the hospital.
According to the 2016 WHO classification of CNS tumors, GS is a variant of IDH-wildtype GBM [3]. GS are unusual malignant primary CNS tumors of the brain, composed of a GBM admixed with a sarcomatous component. The tumor contains a portion that satisfies the histologic criteria for GBM, and a mesenchymal component that may display a variety of morphologies [4]. They affects the adult population in the fourth to seventh decade of life, more predominantly in men [5]. The presenting sign and symptoms varied depending on the location of tumors, the clinical similarities to GBM have led many authors to conclude that these tumors are clinically indistinguishable [5]. The pathogenesis of GS has not yet been established and there has been no detailed study to distinguish between primary GS and secondary GS. Some authors demonstrated p53 mutations, PTEN mutations, p53 nuclear accumulation, p16 deletion, and CDK4 amplications in both tumor areas [567]. In addition, GS were also found to have a fewer number of chromosomes involved in imbalances, suggesting a higher level of genomic stability [8].
Since first description of GS in 1895, almost previous reported articles of GS were located predominantly in the supratentorial area, representing for 1.8–10% of all GBMs [9]. Ng and Poon [10] first reported on a case of radiation induced cerebellar GS in 1990, several case reports about cerebellar GS have been published (Table 1). Only very few cases have been reported about primary GS in infratentorial region [91011121314].
Radiologically, GS in CT scan may appear as a well-defined hyperdense mass with heterogenous or ring enhancement due to a fibrous component. On MRI, GS appears as a heterogeneous mass both in T1- and T2-weighted images, with strong peripheral enhancement and central hemorrhage or necrosis [12]. Dural involvement is not uncommon due to its peripheral location [11]. This case was the first to consider metastasis because the patient had undergone surgery for gastric cancer 15 years ago. In addition, high grade meningioma could not be excluded because of dural invasion. Some image findings were similar to previous reported infratentorial GS, but dural involvement at CPA region was noticed, and its composition was fibrotic and rigid.
Since the incidence of the GS is low, a large-scale study including retrospective & prospective design has not yet been sufficiently investigated. Therefore, treatment of GS is no definitive and is based on the treatment protocol of GBMs. According to the largest recent study for GS, survival was not different for patients with GBM compared with those with GS. In addition, patients treated with trimodality therapy (surgery followed by chemoradiation therapy) had improved survival (12.9 months) compared with those not receiving trimodality therapy (5.5 months). Gross-total resection also improved survival, and MGMT promoter methylation status affected increased survival [15]. But this result is a multicenter study using National Cancer Database in United States, there is a limitation in that miscoding and bias such as missing data may occur. In another single-center study, there was a difference in survival rates compared to previously published studies. Jain et al. [16] reported median overall survival was 5.7 months. The median survival was 1.4 months (range, 0.9–21.4 month) for patients receiving no adjuvant therapy.
In conclusion, we reported a rare case of cerebellar GS. Although extremely rare, GS should be considered in differential diagnosis of tumors arise in infratentorial area. Also, as already known, the prognosis of the GS is poor, but active treatment using multi-modalities can increase the overall survival and obtained better outcomes.
References
1. Kozak KR, Mahadevan A, Moody JS. Adult gliosarcoma: epidemiology, natural history, and factors associated with outcome. Neuro Oncol. 2009; 11:183–191. PMID: 18780813.

2. Galanis E, Buckner JC, Dinapoli RP, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J Neurosurg. 1998; 89:425–430. PMID: 9724117.

3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131:803–820. PMID: 27157931.

4. Zhang G, Huang S, Zhang J, Wu Z, Lin S, Wang Y. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: a clinical study in Chinese patients. J Neurooncol. 2016; 127:355–362. PMID: 26725096.

5. Han SJ, Yang I, Tihan T, Prados MD, Parsa AT. Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol. 2010; 96:313–320. PMID: 19618114.

6. Boerman RH, Anderl K, Herath J, et al. The glial and mesenchymal elements of gliosarcomas share similar genetic alterations. J Neuropathol Exp Neurol. 1996; 55:973–981. PMID: 8800093.

7. Reis RM, Könü-Lebleblicioglu D, Lopes JM, Kleihues P, Ohgaki H. Genetic profile of gliosarcomas. Am J Pathol. 2000; 156:425–432. PMID: 10666371.

8. Actor B, Cobbers JM, Büschges R, et al. Comprehensive analysis of genomic alterations in gliosarcoma and its two tissue components. Genes Chromosomes Cancer. 2002; 34:416–427. PMID: 12112531.

9. Ben Nsir A, Thai QA, Kassar AZ, Ben Said I, Jemel H. Primary cerebellar gliosarcoma with extracranial metastases: an orphan differential diagnosis. World Neurosurg. 2015; 84:2076.e13–2076.e17.

10. Ng HK, Poon WS. Gliosarcoma of the posterior fossa with features of a malignant fibrous histiocytoma. Cancer. 1990; 65:1161–1166. PMID: 2154322.

11. Nitta H, Hayase H, Moriyama Y, Yamashima T, Yamashita J. Gliosarcoma of the posterior cranial fossa: MRI findings. Neuroradiology. 1993; 35:279–280. PMID: 8492894.

12. Moon SK, Kim EJ, Choi WS, Ryu CW, Park BJ, Lee J. Gliosarcoma of the cerebellar hemisphere: a case report and review of the literature. Korean J Radiol. 2010; 11:566–570. PMID: 20808702.

13. Chikkannaiah P, Bharath RD, Sampath S, Santosh V. De novo gliosarcoma occurring in the posterior fossa of a 11-year-old girl. Clin Neuropathol. 2012; 31:389–391. PMID: 22939178.

14. Duan H, Kitazawa K, Yako T, Ichinose S, Kobayashi S, Sudo M. Gliosarcoma in the cerebellopontine angle with rapid tumor growth and intratumoral hemorrhage. World Neurosurg. 2016; 92:580.e17–580.e21.

15. Frandsen J, Orton A, Jensen R, et al. Patterns of care and outcomes in gliosarcoma: an analysis of the National Cancer Database. J Neurosurg. 2018; 128:1133–1138. PMID: 28621623.

16. Jain A, Correia J, Schweder P, McMahon A, Merola J, Aspoas R. Analysis of outcomes of multidisciplinary management of gliosarcoma: a single-center study, 2000-a2013. World Neurosurg. 2017; 106:30–36. PMID: 28642179.
17. Han L, Zhang X, Qiu S, et al. Magnetic resonance imaging of primary cerebral gliosarcoma: a report of 15 cases. Acta Radiol. 2008; 49:1058–1067. PMID: 18766496.

18. Zhang BY, Chen H, Geng DY, et al. Computed tomography and magnetic resonance features of gliosarcoma: a study of 54 cases. J Comput Assist Tomogr. 2011; 35:667–673. PMID: 22082533.
Fig. 1
Brain imaging before surgery. A: Contrast enhanced CT in huge densely enhancing solid mass with peripheral cystic component in the left posterior fossa, attached the left tentorium, with surrounding brain edema. B–D: Gadolinium-enhanced MRI demonstrated 5.6×4.8×3.2 cm sized mass lesion in left posterior cranial fossa, heterogeneously enhanced. There were no diffusion-weighted imaging and gradient echo imaging.
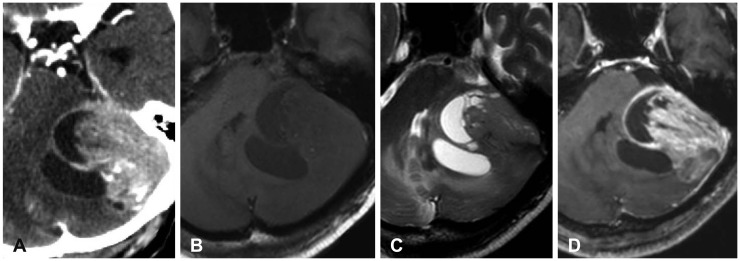
Fig. 2
Histology of gliosarcoma is shown. A: Hematoxylin and eosin stain of the gliomatous component. Glioma cells show polymorphic significantly with tumor necrosis (×100). B: Glial fibrillary acidic protein (GFAP) staining show gliomatous component with strong diffuse GFAP expression (×200). C: Focal GFAP staining is observed between sarcomatous component (×200). D: Diffuse reticulin-rich tumor cells suggest sarcomatous component in reticulin stain (×200).
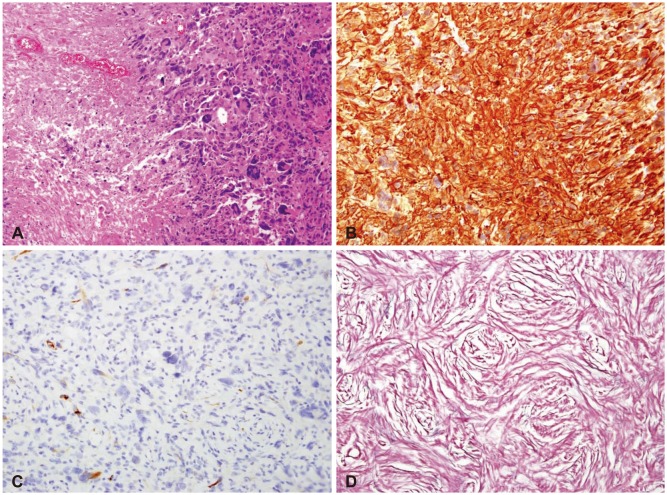
Table 1
Previous reported of infratentorial gliosarcoma
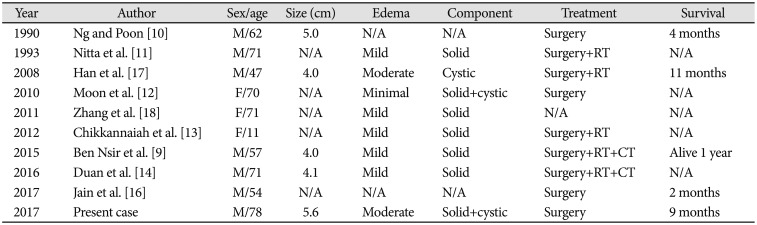
| Year | Author | Sex/age | Size (cm) | Edema | Component | Treatment | Survival |
|---|---|---|---|---|---|---|---|
| 1990 | Ng and Poon [10] | M/62 | 5.0 | N/A | N/A | Surgery | 4 months |
| 1993 | Nitta et al. [11] | M/71 | N/A | Mild | Solid | Surgery+RT | N/A |
| 2008 | Han et al. [17] | M/47 | 4.0 | Moderate | Cystic | Surgery+RT | 11 months |
| 2010 | Moon et al. [12] | F/70 | N/A | Minimal | Solid+cystic | Surgery | N/A |
| 2011 | Zhang et al. [18] | F/71 | N/A | Mild | Solid | N/A | N/A |
| 2012 | Chikkannaiah et al. [13] | F/11 | N/A | Mild | Solid | Surgery+RT | N/A |
| 2015 | Ben Nsir et al. [9] | M/57 | 4.0 | Mild | Solid | Surgery+RT+CT | Alive 1 year |
| 2016 | Duan et al. [14] | M/71 | 4.1 | Mild | Solid | Surgery+RT+CT | N/A |
| 2017 | Jain et al. [16] | M/54 | N/A | N/A | N/A | Surgery | 2 months |
| 2017 | Present case | M/78 | 5.6 | Moderate | Solid+cystic | Surgery | 9 months |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download