Abstract
Objective
The standard antenatal screening method for Group B Streptococcus (GBS) has not been established yet. Therefore, many practitioners in South Korea offer GBS screening to all pregnant women without solid clinical evidence. The aim of this study was to compare the rates of early onset neonatal sepsis (EONS) according to two different antenatal GBS screening methods – risk-based versus universal screening.
Methods
This is a retrospective cohort study from January 2014 to April 2017. The study period was divided into two 16-month periods: from January 2014 to April 2015 in which risk-based screening was performed (period 1), and from January 2016 to April 2017 in which universal screening was performed (period 2). We compared the rates of EONS caused by GBS and other bacterial species between the two periods.
Results
1,301 neonates from 1,293 deliveries and 924 neonates from 913 deliveries were enrolled in period 1 and period 2, respectively. Suspected or culture-proven EONS caused by any organisms were more frequently observed in period 2 (0.7% in period 1 vs. 1.8% in period 2, P=0.013). The causative organism was not confirmed by culture in most cases, except for GBS, Escherichia coli, and Enterococcus. Intrapartum administration of antibiotic prophylaxis (IAP) was more frequently performed in period 2 (10.9% in period 1 vs. 21.5% in period 2, P<0.001).
Early onset neonatal sepsis (EONS) by Group B Streptococcus (GBS) is a leading cause of life threatening infection in newborns causing sepsis, pneumonia, meningitis and death.12 It is important to realize the risk factors for neonatal sepsis in order to establish optimal prevention and management strategies. Intrapartum administration of antibiotic prophylaxis (IAP) has shown to lower the incidence of early onset neonatal GBS infection.345 Screening for women requiring IAP has been done via one of two approaches, universal screening and risk-based approach in which women receive IAP based on the presence of risk factors.16
Universal screening is practiced in the United States and Canada1789 and it is also recommended with some modifications in many European countries.10 On the other hand, risk-based approach is recommended in Denmark, Netherlands and the United Kingdom.111 In South Korea, however, there still is a lack of evidence as to which approach is more optimal to prevent EONS.
The epidemiology of GBS varies geographically.11213 Studies have shown that approximately 20% of pregnant women in the United States are infected with GBS.14 In Europe, colonization rates are reported to be about 19% to 29% in the Eastern region, 11% to 21% in the Western region, and 6% to 32% in the Southern region.15 GBS infection in South Korea has also been reported to be low (Table 1).13 In South Korea, the prevalence of maternal GBS colonization was 0.3% to 5.9% before 2010.1617181920 However, recent study published in 2010 reported the increased prevalence of maternal GBS in Korean hospitals as 8%, ranging from 2.0% to 10.0%.21
GBS is the leading cause of neonatal sepsis and meningitis since the late 1990.22 However, only limited data about neonates with GBS infections are available for many Asian countries including South Korea, and most of the data have been derived from cross-sectional studies.2324 Moreover, there are no clinical guidelines for GBS prevention and only few data are available regarding the risk factors of GBS colonization. This is probably due to the low GBS prevalence rates in South Korea. In this regard, it is warranted to investigate the recent rates of GBS infection in Korean pregnant women.
Given this background, we aimed to investigate the changes of EONS after the adoption of universal GBS screening instead of risk-based screening in Samsung Medical Center. The main hypothesis of the study was that the universal screening method would not decrease the rates of EONS compared to the risk-based screening methods. We examined the rates of GBS colonization, rates of EONS and causative organisms of EONS before and after the adoption of universal screening. We also evaluated the clinical risk factors for GBS colonization. To the best of our knowledge, this is one of the largest studies to compare EONS before and after the adoption of universal GBS screening in South Korea.
This is a retrospective cohort study of full-term pregnant women who underwent antenatal risk-based or universal GBS screening. We compared the maternal and neonatal outcomes between the two periods of the same duration: period 1 of risk-based approach from January 2014 to April 2015 and period 2 of universal screening from January 2016 to April 2017.
In period 1, positive urine culture at any time during pregnancy, GBS bacteriuria and birth of a previous infant with GBS sepsis were considered as risk factors for GBS EONS.25 Intrapartum risk factors for neonatal infection were intrapartum maternal fever (>38℃) and prolonged rupture of membranes more than 18 hours. IAP was carried out in women with any one of these risk factors. Intrapartum period was defined as the time between the beginning of contractions that caused cervical dilatation and the delivery of the newborn and placenta. In period 2, universal GBS screening was done between 35 to 37 weeks of gestation excluding scheduled elective cesarean section.
It has been reported that preterm delivery is associated with high risk of GBS sepsis in neonates. The odds ratio of EONS due to GBS was reported to be 4.8 in preterm delivery when compared to neonates delivered after 37 weeks of gestation.25 However, the purpose of this study was the screening for the prophylactic antibiotics, those women with preterm delivery were excluded from this study.
Without using speculum, specimens were obtained from lower third of vagina and perianal areas except anus by a pair of rayon swabs. Then the swabs were collected with a Copan Venturi Transystem collection device (Copan innovation, Corona, CA, USA), which consisted of Liquid Stuart transport medium. Blood agar plate (BAP) (Shinyang chemical, Seoul, Korea), MacConkey agar and Thayer-Martin agar (Hanilkomed, Seongnam, Korea) were used for isolation. Samples were inoculated onto media for 18–24 hours at 35℃ in 5% CO2 gas chamber. GBS was identified by several distinguishing features, including characteristic patterns of beta-hemolysis, colony morphology on BAP and Gram-stained cell morphology. Colonies on BAP resembling GBS were identified by direct use of matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (VITEK MS, Marcy-l'Étoile, France).
A portion of fresh colony was smeared onto a Vitek MS DS (VITEK MS) target slide and the preparations were overlaid with 1 mL matrix solution (a saturated solution of a-cyano-4-hydroxycinnamic acid in 50% acetonitrile and 2.5% trifluoroacetic acid). After drying, the target plate was loaded into the Vitek MS mass spectrometer and air-dried at room temperature for 1 to 2 minutes. For a calibration and internal identification control, the GBS strain (ATCC 13813; ATCC, Manassas, VA, USA) was inoculated on the calibration spots. The 500 shots from different positions of each spot were collected by the mass spectrometer with the Acquisition Station software package (VITEK MS). Generated mass fingerprints were processed by the computer engine, and the advanced spectrum classifier algorithm automatically identified the organism by comparing the obtained peaks. A confidence value was calculated and this number represents the specific peaks between the generated spectrum and the database spectra.26
IAP was carried out during labor according to the Centers for Disease Control (CDC) guideline (i.e., all colonized women were offered intrapartum antibiotics at the time of labor onset or rupture of membranes. GBS bacteriuria or positive GBS EONS history were also indications for IAP).7 The first generation of cephalosporin (Cefazolin; Chongkundang Pharmaceutical corporation, Seoul, Korea) was used for IAP because pharmacologic data suggest its effectiveness in transplacental perfusion and use in pregnancy.7 Two grams of cefazolin were loaded intra venously (IV), followed by 1 gram of maintenance dose intravenous infusion every 8 hours until delivery. Patients who were allergic to cephalosporin were administered ampicillin instead (2 grams IV for loading dose, followed by 1 gram IV every 4 hours). Vancomycin and clindamycin were reserved for penicillin-allergic women at high risk of anaphylaxis with a history of angioedema, respiratory distress, or urticaria following administration of penicillin or cephalosporin.
Maternal and neonatal data were reviewed as following: maternal age, parity, use of assisted reproductive technology (ART), gestational diabetes (GDM), intrapartum risk factors (maternal intrapartum fever >38℃ and prolonged rupture of membranes longer than 18 hours), mode of delivery, neonatal birth weight, admission to the neonatal intensive care unit (NICU) rate, EONS, and neonatal death. Neonatal sepsis was diagnosed by clinical findings and the presence of bacteria or fungus in the blood culture. Neonates who presented clinical symptoms only were classified as “suspected sepsis”, whereas those with positive blood culture were classified as “proven sepsis”. EONS was defined as neonatal sepsis diagnosed at 0–6 days of life, proven by culture or suspected and treated in NICU. We analyzed the relation of the GBS colonization with the following possible risk factors using multiple logistic regression analysis in period 2: maternal age (subdivided in 5 groups; <25, 25–29, 30–34, 35–39, and ≥40 years of age), body mass index (BMI), parity, use of ART, twin pregnancy, GDM.
We used the Mann-Whitney's U test for continuous variables and the Fischer's exact test or Chi-square test for categorical variables. Multivariable analysis was performed using logistic regression analysis. P-value less than 0.05 was considered statistically significant. The statistical analysis was carried out using the SAS software (version 9.4; SAS Institute, Cary, NC, USA).
A total 2,206 of pregnant women were included in the study: period 1 of risk-based approach (n=1,293; from January 2014 to April 2015) and period 2 of universal screening (n=913; from January 2016 to April 2017). In period 2, 795 out of 913 (87%) women underwent universal GBS screening (Fig. 1).
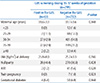
The baseline maternal characteristics of both periods are described in Table 2. The maternal age and BMI at delivery were significantly higher in period 2 than period 1 (maternal age 32.7±3.6 in period 1 vs. 33.1±3.6 in period 2, P=0.038; BMI 25.2±3.1 in peroid 1 vs. 25.7±3.4 in peroid 2, P=0.004)
There were no significant differences between the two periods with regard to gestational age at delivery, parity, the history of ART, GDM, and twin pregnancy. Among the maternal risk factors of the neonatal GBS infection, intrapartum fever (>38℃) was significantly higher in period 2 (Table 3). The IAP was more frequently administered during period 2 than period 1.
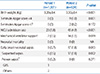
The neonatal outcomes are summarized in Table 4. The birth weight, small for gestational age (SGA), neonates with 1-minute Apgar score <4, and the NICU admission rate were significantly higher in period 2. EONS rate was 0.7% in period 1 and 1.8% in period 2. There was only one case of GBS culture-proven sepsis (0.2%) in period 1. However, there was no culture-proven GBS case in period 2 even though the overall EONS rate was higher in period 2.
In period 2, 795 (795/913, 87.1%) women underwent universal GBS screening (Fig. 1). One hundred eighteen women (13%) were not able to undergo GBS screening due to the following reasons: women were transferred from other hospitals after the onset of labor or emergent cesarean section was performed immediately after the arrival at the present institution.
The number of women whose swab culture contained normal vaginal flora only was 499 (62.8%) in period 2. Our institution (Samsung Medical Center) designated normal flora as predominant non-pathogen such as coagulase-negative Staphylococci, Streptococcus viridans group, Corynebacterium species, and Enterococcus species. GBS colonization was observed in 63 (7.9%) in period 2. In the distribution of isolated microorganisms, Escherichia coli accounted for 50.0%, GBS for 21.2%, Candida for 16.9%, Staphylococcus aureus for 4.3% (Fig. 2). Screening results were not available in 28 women (3.5%) before delivery and IAP was not administered in 11 women (1.4%) due to precipitous delivery.
The multivariable analysis of the maternal characteristics in regard of GBS colonization is shown in Table 5. Maternal age, BMI, parity, twin pregnancy, ART and GDM were not different in women who were positive or negative for GBS colonization in period 2.
The incidence of GBS varies according to geographic regions and the recommendation for prevention of GBS EONS varies greatly worldwide.14121327 The prevalence of GBS colonization in South Korea has been examined in many studies during the last decades.1617181920 Although the GBS colonization rates slightly increased from 1995 to 2011, the rates have been stationary at about 8% after 2006.>181920
In general, both the routine screening and the risk-based screening can be used to select the candidates of IAP. Recently, the former was recommended by the American College of Obstetrics and Gynecologists (ACOG)8 and CDC guidelines.7 However, it has several disadvantages including high cost, false negative screening results, risk of penicillin-induced anaphylaxis, and selection of resistant bacterial strains in newborns. Additionally, the increased use of antibiotics invoked changes in GBS serotypes resulting in resistant strains to clindamycin and erythromycin, even fluoroquinolone in South Korean studies.2829 Contrary to the ACOG guidelines, the Royal College of Obstetricians and Gynecologists guidelines advocates the risk-based approach due to the cost-effectiveness because the incidence of GBS neonatal sepsis in the United Kingdom is too low and the routine screening has not been proven to be superior to its counterpart in the randomized trials.11 However, the risk-based approach may miss preventable GBS sepsis if the incidence is high.27 Consequently, there are controversies on optimal preventive strategy especially in the regions with high prevalence of GBS.2730
Our hypothesis in this study was that the universal screening would not decrease the rates of GBS EONS. The EONS incidences in both period 1 and period 2 were similar. However, the use of IAP almost doubled since the universal screening method was adopted. Therefore, if the same results were observed in larger population, universal screening method would only have increased the antibiotic usage without reducing EONS.
In our results, not only the use of IAP but also fever >38℃ during labor was significantly higher in period 2 compared to period 1. Recent studies of intrapartum fever in full term gestations showed that nulliparity, duration of the first stage of labor longer than 720 minutes, duration of the second stage longer than 120 minutes, duration of membrane rupture longer than 240 minutes, frequent vaginal exams during labor, the use of oxytocin, and meperidine were all associated with intrapartum fever.31 Considering that the rates of cesarean delivery after trial of vaginal delivery was higher in period 2 (13.2% in period 1 vs. 20.3% in period 2, P<0.001; Table 2), failure of vaginal delivery may be related to prolonged second stage of labor and consequently resulted in intrapartum fever. Additionally, oxytocin was used in the presence of labor dystocia for labor augmentation and meperidine for pain control, which may be another potential reasons for intrapartum fever in our study.
There were 116 cases (15.9%) of IAP administration in period 2 that were not positive for GBS culture. Those cases were either GBS screening result was not reported until delivery or was reported negative but IAP was administered according to physician's discretion. There were also 11 cases (17.5%) of no IAP administration with GBS positive in period 2 (Table 5). Three of these cases were those in which the results were not reported after GBS culture by the time of delivery. The remaining eight cases were due to errors.
Compared with the proven EONS of three cases (0.2%) in period 1, proven EONS was 0 case (0%) in period 2 in neonatal outcomes. On the contrary, suspected sepsis and the admission rates to NICU increased in period 2. The reason for the increased number of suspected sepsis is unclear. However, low levels of neonatal bacteremia or only small amount of blood acquired from neonates may be one explanation for the high number of suspected sepsis.32 In addition, maternal antibiotic treatment before or during labor may theoretically mask bacteremia in newborn neonates. Considering the proportion of high-risk pregnancy in our tertiary care center, it seems clear that the increased proportion of SGA and 1 minute Apgar score <4 in period 2 might have led to increase in NICU admission rates.
The limitation of present study is the low statistical power. Considering the primary outcome as EONS rates, the effect size is 9 (0.7%) in period 1 and 17 (1.8%) in period 2 (Table 4). Then the power of study is 65% with type 1 error of 0.05 using Chi-square test. Considering that the present study was conducted at a tertiary medical center and the nature of retrospective study, the results observed in the present study may contain selection bias. Even after the adoption of the universal screening, there were still a few problems such as the lack of screening test due to transfers from other hospitals after the onset of labor, unavailability of GBS screening results before delivery and the failure of administration of IAP due to precipitous labor. Another limitation of the study is the specimen collection method. According to the ACOG guideline, specimens were taken from the lower vagina and rectum (through the anal sphincter) for maximum recovery. Since our specimens were obtained from perianal area, not from the rectum, the actual rate of GBS colonization may be higher than that of our study.
In conclusion, the rate of maternal GBS colonization was 7.9% similar to previous reports. Even after the adoption of universal GBS screening, there was no case of proven EONS due to GBS in period 2. In this aspect, the primary goal of GBS screening was achieved during the universal screening period. However, EONS rate (suspected and proven) and the use of IAP increased significantly. Considering the overuse of intra-partum antibiotics, it is unclear whether routine GBS screening would be beneficial or cost-effective with regard to prevention of neonatal infection in Korea. Further large studies are required to clarify the most suitable strategy for prevention of EONS due to GBS in South Korea.
Figures and Tables
Fig. 1
The flow chart of universal GBS screening in the period 2. Excluding fetal major malformation and preterm delivery, universal GBS screening was applicable to 913 women. Among them, 795 women underwent universal GBS screening.

Fig. 2
Isolated microorganisms in the universal GBS screening period. The rate of GBS colonization was found to be 7.9%. Other than GBS, E. coli and Candida accounted for 18.6% and 6.3% of isolated organism, respectively. E. coli, Escherichia coli; GBS, Group B Streptococcus; S. aureus, Staphylococcus aureus.

Table 3
Maternal Risk Factors of the Neonatal GBS Infection and the IAP Administration in the Period 1 and the Period 2

References
1. Homer CS, Scarf V, Catling C, Davis D. Culture-based versus risk-based screening for the prevention of group B streptococcal disease in newborns: a review of national guidelines. Women Birth. 2014; 27:46–51.

2. Sun HD, Su WH, Chang WH, Wen L, Huang BS, Wang PH. Rupture of a pregnant unscarred uterus in an early secondary trimester: a case report and brief review. J Obstet Gynaecol Res. 2012; 38:442–445.

3. Money D, Allen VM. Infectious Diseases Committee. The prevention of early-onset neonatal group B streptococcal disease. J Obstet Gynaecol Can. 2013; 35:939–948.

4. Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013; 31:Suppl 4. D20–D26.

5. Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013; 121:570–577.

6. Kurz E, Davis D. Routine culture-based screening versus risk-based management for the prevention of early-onset group B Streptococcus disease in the neonate: a systematic review. JBI Database System Rev Implement Rep. 2015; 13:206–246.

7. Verani JR, McGee L, Schrag SJ. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010; 59:1–36.
8. American College of Obstetricians and Gynecologists. ACOG committee opinion: number 279, December 2002. Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2002; 100:1405–1412.
9. Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, et al. Evaluation of universal antenatal screening for group B Streptococcus. N Engl J Med. 2009; 360:2626–2636.

10. Berardi A, Di Fazzio G, Gavioli S, Di Grande E, Groppi A, Papa I, et al. Universal antenatal screening for group B Streptococcus in Emilia-Romagna. J Med Screen. 2011; 18:60–64.

11. Prevention of. green-top guideline No. 36. BJOG. 2017; 124:e280–e305.
12. Melin P, Efstratiou A. Group B streptococcal epidemiology and vaccine needs in developed countries. Vaccine. 2013; 31:Suppl 4. D31–D42.

13. Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012; 379:547–556.

14. Anderson EL, Cole JN, Olson J, Ryba B, Ghosh P, Nizet V. The fibrinogen-binding M1 protein reduces pharyngeal cell adherence and colonization phenotypes of M1T1 group A Streptococcus. J Biol Chem. 2014; 289:3539–3539.

15. Barcaite E, Bartusevicius A, Tameliene R, Kliucinskas M, Maleckiene L, Nadisauskiene R. Prevalence of maternal group B streptococcal colonisation in European countries. Acta Obstet Gynecol Scand. 2008; 87:260–271.

16. Uh Y, Jang IH, Yoon KJ, Lee CH, Kwon JY, Kim MC. Colonization rates and serotypes of group B streptococci isolated from pregnant women in a Korean tertiary hospital. Eur J Clin Microbiol Infect Dis. 1997; 16:753–756.

17. Hong JS, Choi CW, Park KU, Kim SN, Lee HJ, Lee HR, et al. Genital group B Streptococcus carrier rate and serotype distribution in Korean pregnant women: implications for group B streptococcal disease in Korean neonates. J Perinat Med. 2010; 38:373–377.

18. Bang SM, Seo JW, Park KU, Kim SJ, Kim K, Kim SH, et al. Molecular cytogenetic analysis of Korean patients with Waldenström macroglobulinemia. Cancer Genet Cytogenet. 2010; 197:117–121.

19. Woo HI, Kim HJ, Lee SH, Yoo KH, Koo HH, Kim SH. Acute myeloid leukemia with complex hypodiploidy and loss of heterozygosity of 17p in a boy with Fanconi anemia. Ann Clin Lab Sci. 2011; 41:66–70.
20. Yook JH, Kim MY, Kim EJ, Yang JH, Ryu HM, Oh KY, et al. Risk factors associated with group B Streptococcus resistant to clindamycin and erythromycin in pregnant Korean women. Infect Chemother. 2013; 45:299–307.

21. Lee BK, Song YR, Kim MY, Yang JH, Shin JH, Seo YS, et al. Epidemiology of group B Streptococcus in Korean pregnant women. Epidemiol Infect. 2010; 138:292–298.

22. Rhie K, Choi EH, Cho EY, Lee J, Kang JH, Kim DS, et al. Etiology of invasive bacterial infections in immunocompetent children in Korea (2006-2010): a retrospective multicenter study. J Korean Med Sci. 2018; 33:e45.

23. Konrad G, Katz A. Epidemiology of early-onset neonatal group B streptococcal infection: implications for screening. Can Fam Physician. 2007; 53:1054–1055.
24. Lee JH, Kim SM, Lee HS, Kim SY, Choi SD, Sung IK, et al. A clinical study of group B streptococcal infection: five years experience. Neonatal Med. 2003; 10:226–234.
25. Gerdes JS. Diagnosis and management of bacterial infections in the neonate. Pediatr Clin North Am. 2004; 51:939–959.

26. Luo Y, Siu GK, Yeung AS, Chen JH, Ho PL, Leung KW, et al. Performance of the VITEK MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for rapid bacterial identification in two diagnostic centres in China. J Med Microbiol. 2015; 64:18–24.

27. Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002; 347:233–239.

28. Ki M, Srinivasan U, Oh KY, Kim MY, Shin JH, Hong HL, et al. Emerging fluoroquinolone resistance in Streptococcus agalactiae in South Korea. Eur J Clin Microbiol Infect Dis. 2012; 31:3199–3205.

29. Seo YS, Srinivasan U, Oh KY, Shin JH, Chae JD, Kim MY, et al. Changing molecular epidemiology of group B Streptococcus in Korea. J Korean Med Sci. 2010; 25:817–823.

30. Gibbs RS, Schrag S, Schuchat A. Perinatal infections due to group B streptococci. Obstet Gynecol. 2004; 104:1062–1076.

31. Burgess APH, Katz JE, Moretti M, Lakhi N. Risk factors for intrapartum fever in term gestations and associated maternal and neonatal sequelae. Gynecol Obstet Invest. 2017; 82:508–516.

32. Klingenberg C, Kornelisse RF, Buonocore G, Maier RF, Stocker M. Culture- negative early-onset neonatal sepsis - at the crossroad between efficient sepsis care and antimicrobial stewardship. Front Pediatr. 2018; 6:285.

33. Choi KU, Koh SK, Lee JY, Park JH, Hwang SO, Lee BI, et al. Clinical significance of group B streptococcal infection in pregnant women. Korean J Obstet Gynecol. 2002; 45:811–815.
34. Kim MW, Jang HO, Chang DY, Cho JR, Kim YA, Choi HM, et al. Group B streptococcal colonization rate in Korean pregnant women. Korean J Obstet Gynecol. 2006; 49:337–344.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download