Abstract
Different surgical procedures have been described for the treatment of colorectal liver metastases. The appropriate surgical approach depends, among many other factors, on the relationship between liver metastases and suprahepatic veins. If possible, the detachment of colorectal liver metastasis from suprahepatic veins is a good alternative liver parenchyma sparing technique. In this study, we describe a new two-step wedge liver resection technique for colorectal liver metastases located between suprahepatic veins. Prior to resection, intraoperative ultrasound is employed in order to plan and guide both steps. Initially, we place stitches and resect a cylindrical piece of normal liver parenchyma above the tumor and suprahepatic veins. Next, we place stitches on the future specimen located between suprahepatic veins, then resect it. The main advantages of this procedure are the good visualization and vascular control that may be achieved during the detachment of the tumor from suprahepatic veins. We call this procedure “zoom resection” because its dynamics are similar to the workings of a photograph camera's telescopic system. We present the case of a 55-year-old patient diagnosed with multiple colorectal liver metastases, one of which was resected through this technique.
Hepatic resection for colorectal liver metastases (CLM) is the treatment of choice in those cases which are resectable. The selection of the best surgical approach is linked to the number, distribution,1 and relationship of the CLM with the hepatic vascular pedicles.2 Because future hepatic remnant is another important aspect to take into account, wedge hepatic resections are frequently employed in order to spare normal liver parenchyma.234 However, CLMs close to vascular pedicles can sometimes require major hepatic resections5 or complex vascular reconstructions.26 In this report, we describe a simple and easy-to-perform two-step wedge liver resection technique applied to the detachment of a CLM located between the left and middle suprahepatic veins (SHVs) in a 55-year-old patient.
We present a 55-year-old patient with a history of obesity and psychiatric anxiety disorder who was diagnosed with a synchronic right colorectal tumor and bilateral CLM. A computed tomography (CT) scan showed a right colorectal tumor and at least four CLM. Our multidisciplinary Oncology Committee decided to start by treating the colorectal tumor. A right laparoscopic hemicolectomy was performed in April 2017. Pathological examination revealed a 5 cm-sized colorectal adenocarcinoma and five of 27 lymph nodes infiltrated by adenocarcinoma (T3 N2 M1). Thereafter, three cycles of adjuvant chemotherapy with capecitabine and oxaliplatinum were completed. After that, magnetic resonance imaging (MRI) and positron emission tomography/CT showed incomplete clinical responses to chemotherapy and the persistence of at least two CLM. A hepatic resection was then planned. As is typically done, we first mobilized the liver and performed an intraoperative ultrasound (IOUS). At the moment of surgery, we found five CLM, four of them in segments II (n=2), III (n=1), and IVa (n=1) and only one very large CLM in the right hemi-liver. Next, we performed three superficial wedge liver resections in order to treat the CLM located in segments II and III. In contrast, segment IVa CLM was deeper and located very close to and between the left and middle SHVs. In this case, we employed a new two-step wedge liver resection technique (see below) to detach the segment IVa CLM from SHVs. A zero millimeter margin was achieved. We then ligated and cut the right portal branch. The patient was discharged seven days later without complications.
A volumetric liver CT scan conducted 45 days later showed a unique giant 15 cm-sized right CLM in contact with inferior vena cava, a left hemi-liver volume (segments I-II-III-IV) of 854 ml (38%), and a total liver volume of 2214 ml. The patient's weight was 95.5 kg.
Two months after the first surgery, we performed a right hepatectomy in block with a subtotal resection of the right hemi-diaphragm. The latter was required due to tumor infiltration. The patient was discharged ten days later without complications.
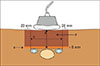
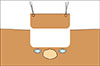
To begin, IOUS was performed. A segment IVa CLM was located between the left and middle SHVs, and we assessed no evidence of tumor vascular infiltration (Fig. 1). We then proceeded to measure the distance between the liver surface and the SHVs's upper edge. Next, Glisson's capsule was marked with electrocautery 20 millimeters away from the outer edges of both SHVs (Fig. 2). After that, the marked area was anchored with stitches that were placed surrounding it. A cylindrical piece of normal liver parenchyma above the CLM and SHVs was then resected. A 5 millimeter resection margin was left above the SHVs (Fig. 3).
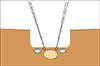
Next, an ultrasonography transducer was placed on the surface of the wedge resection area in order to mark the location of the segment IVa CLM. Finally, we placed stitches on the specimen located between the SHVs and resected it (Fig. 4). Because the segment IVa CLM was so close to both SHVs, the skeletonization of both the middle and left SHVs was required. A zero millimeter margin was achieved (Fig. 5, 6).
Given that the dynamics of the two-step wedge liver resection technique are similar to the workings of a photograph camera's telescopic system, we have named this technique “zoom resection”.
Hepatic resection for CLM is the best treatment option in cases which are resectable.1 The selection of the optimal surgical approach is linked, among other aspects, to the relationship of the liver metastases with the great hepatic pedicles25 R0 resection (>1 mm margin) should be the main objective of all CLM resections7 However, it sometimes implies performing major hepatectomy, which is not always possible in cases of bilateral CLM disease. Detachment of CLMs (by definition a R1 resection) from major intrahepatic vessels has been systematically attempted in order to avoid major hepatectomy and increase resectability.8 In 2008, de Haas et al.9 first reported that there were no negative prognostic impacts of positive surgical margins. Most recently, Viganò et al.10 showed that there were no differences in overall survival and local recurrence between R0 resection and R1 vascular margin (R1vasc). In contrast, R1 parenchymal margin (R1Par) had poor outcomes in a comparative study. Bearing these studies in mind, we believe that the R1Vasc accomplished in our patient with the “zoom resection” technique was a good surgical treatment option. Having said that, we must note that we employed the Cavitron ultrasonic aspirator (CUSA) to perform CLM detachment from SHVs, and this transection technique may distort the edge of the margin by aspirating a few mm of surrounding hepatic tissue. Therefore, the pathological assessment may have underestimated the width of the margin. This issue has been previously reported.11
Future hepatic remnant is another important aspect to take into account. In this sense, hepatic parenchyma-sparing techniques, such as wedge hepatic resection, have been reported.3412 Prof. De Santibañes et al. have described a simple and useful technique for the resection of CLM.4 The principle of traction and countertraction described by those authors was applied in our technique. However, that technique was described for the purpose of carrying out superficial CLM resections. The difference between that technique and ours is the fact that we added an extra step so as to facilitate vascular control in deeper CLM resections. The concept of the resection of a portion of normal liver parenchyma prior to the resection of a tumor had been previously described by Horton et al.13 They proposed a left lateral segmentectomy prior to resecting a deep CLM located under the middle hepatic vein. They then, suggest carrying out a non-anatomical liver resection through the cut surface of the liver. Although this technique could be a useful tool in some cases, the problem is that it is not always possible to perform a left lateral segmentectomy in cases of bilateral CLM disease. In fact, it would have been impossible to perform it on our patient due to insufficient future liver remnant.
To summarize, we believe that “zoom resection” may be an easy and simple technique for detaching deep CLM located between SHVs. We hope that this case report on the technique will serve as a starting point for further studies.
Figures and Tables
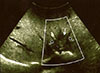
Fig. 1
Intraoperative ultrasonography shows the anatomical relationship between the IVa colorectal liver metastases (white arrow) and suprahepatic veins (SHVs) (black arrows).
References
1. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999; 230:309–318.
2. Urbani L, Colombatto P, Balestri R, Licitra G, Leoni C, Forfori F, et al. Techniques of parenchyma-sparing hepatectomy for the treatment of tumors involving the hepatocaval confluence: a reliable way to assure an adequate future liver remnant volume. Surgery. 2017; 162:483–499.
3. Donadon M, Torzilli G. From mesohepatectomy to mini-mesohepatectomy: evolving the concept of resectability of hepatic tumors at the hepatocaval confluence. Dig Surg. 2011; 28:109–113.

4. de Santibañes E, Sánchez Clariá R, Palavecino M, Beskow A, Pekolj J. Liver metastasis resection: a simple technique that makes it easier. J Gastrointest Surg. 2007; 11:1183–1187.

5. van Dam RM, Lodewick TM, van den Broek MA, de Jong MC, Greve JW, Jansen RL, et al. Outcomes of extended versus limited indications for patients undergoing a liver resection for colorectal cancer liver metastases. HPB (Oxford). 2014; 16:550–559.

6. Aoki T, Sugawara Y, Imamura H, Seyama Y, Minagawa M, Hasegawa K, et al. Hepatic resection with reconstruction of the inferior vena cava or hepatic venous confluence for metastatic liver tumor from colorectal cancer. J Am Coll Surg. 2004; 198:366–372.
7. Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, Andreatos N, Tzanninis IG, Sasaki K, et al. Impact of surgical margin width on recurrence and overall survival following R0 hepatic resection of colorectal metastases: a systematic review and meta-analysis. Ann Surg. 2018; 267:1047–1055.
8. Torzilli G, Montorsi M, Donadon M, Palmisano A, Del Fabbro D, Gambetti A, et al. “Radical but conservative” is the main goal for ultrasonography-guided liver resection: prospective validation of this approach. J Am Coll Surg. 2005; 201:517–528.

9. de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008; 248:626–637.
10. Viganò L, Procopio F, Cimino MM, Donadon M, Gatti A, Costa G, et al. Is tumor detachment from vascular structures equivalent to R0 resection in surgery for colorectal liver metastases? An observational cohort. Ann Surg Oncol. 2016; 23:1352–1360.

11. Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005; 241:715–722.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download