Abstract
It is expected that a combination of transcatheter arterial chemoembolization (TACE) with stereotactic body radiation therapy (SBRT) may induce synergistic therapeutic effects in hepatocellular carcinoma (HCC), which would result in a high rate of complete therapeutic response. In this study, we present the 5-year clinical course of a patient who had HCC at the caudate lobe, which was treated with TACE and SBRT. A 53-year-old male was diagnosed with an 8 cm-sized HCC at the caudate lobe with compression of the inferior vena cava (IVC). For fear of pulmonary metastasis, we decided to perform sequential TACE-radiotherapy instead of upfront hepatectomy, although the tumor appeared resectable. The first session of TACE, SBRT with 12 fractions, and the second session of TACE were sequentially performed. The patient was administered metformin for chemoprevention. Over the course of a 5-year follow-up, there was no evidence of HCC recurrence. We reported the clinical sequence of a patient showing complete therapeutic response of HCC at the caudate lobe after a combination of TACE and radiotherapy. This type of combined locoregional treatment can be a therapeutic option for HCC at the caudate lobe with marginal resectability.
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and is one of the leading causes of cancer-related deaths.12 Hepatic resection (HR) is considered to be the preferred treatment method for HCC but is also considered to be a challenging surgical procedure in the presence of liver cirrhosis. HR resection for HCC encircling the retrohepatic inferior vena cava (IVC) increases the risk of an intraoperative tumor spread during the handling of the hypervascular mass.3456
Transcatheter arterial chemoembolization (TACE) represents one of the locoregional therapies for HCC. TACE often improves outcomes in patients with unresectable HCCs, but it has not been considered a curative treatment because of high recurrence rates. We previously reported that preoperative TACE for resectable HCC may adversely Transcatheter arterial chemoembolization (TACE) represents one of the locoregional therapies for HCC. TACE often improves outcomes in patients with unresectable HCCs, but it has not been considered a curative treatment because of high recurrence rates. We previously reported that preoperative TACE for resectable HCC may adversely affect post-resection prognosis, irrespective of pathological responses. Thus, we suggested that preoperative TACE should be avoided for patients with resectable small HCCs.7
With recent advances in the radiotherapy techniques, stereotactic body radiation therapy (SBRT) has been considered an alternative treatment option for a small HCC not suitable for hepatic resection and other locoregional treatments.8910 More recently, volumetric-modulated arc therapy, one of the most sophisticated linear accelerator-based treatment modalities, has become available even with gated delivery. This method allows dose rate-changing intensity modulation with gantry rotation and may provide more conformal dose distribution while reducing treatment delivery time and monitor units.
It is expected that a combination of TACE with SBRT may induce synergistic therapeutic effects, which would result in a high rate of complete therapeutic response. In the present study, we report the 5-year clinical course of a patient who had HCC at the caudate lobe and was treated with TACE and SBRT.
A 53-year-old male was admitted for examination of a liver mass at the caudate lobe. The mass was first detected during a routine health screening 3 years before. It was 7 cm in size at that time with no elevation of tumor markers (Fig. 1). During a follow-up with liver ultrasonography, its size had increased very slowly. He had a past history of hepatitis B virus (HBV) infection. During the precedent follow-up, HBV surface antigen (HBsAg) became seronegative with the appearance of anti-HBs. At admission, HBV DNA was not detectable on polymerase chain reaction assays; Serum alpha-fetoprotein (AFP) was 3.2 ng/ml and prothrombin-induced by vitamin K absence or antagonist-II (PIVKA-II) was 988 mAU/ml.
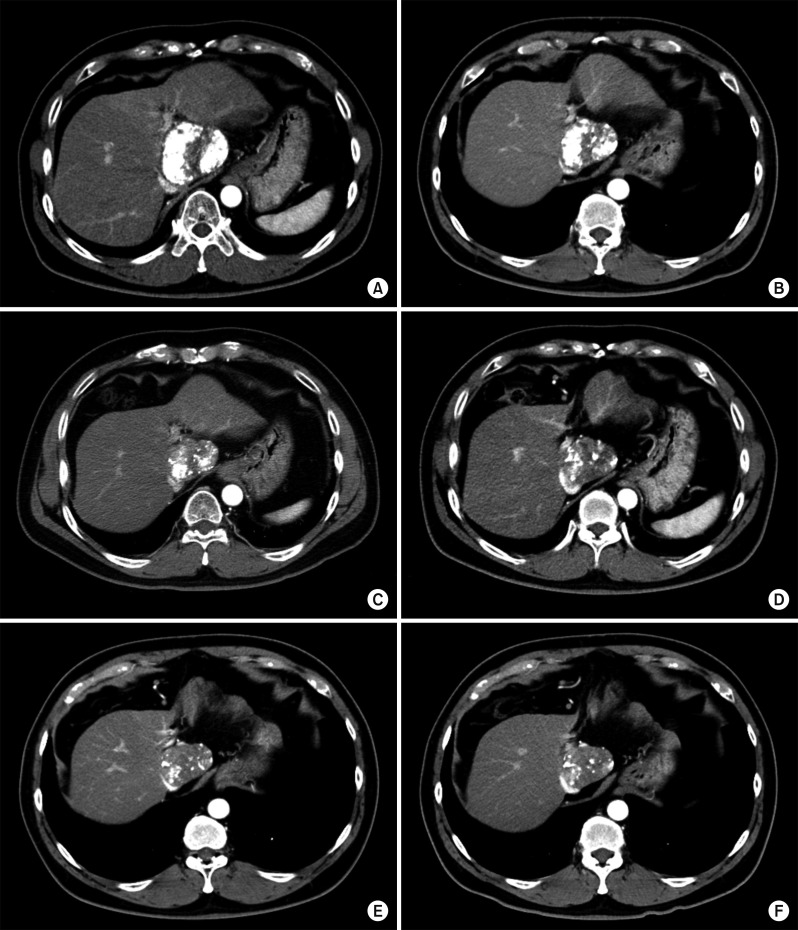
Computed tomography (CT) revealed an 8 cm-sized hypervascular mass with calcification at the caudate lobe of the liver, which was abutted with the IVC (Fig. 2). Magnetic resonance imaging showed a hypervascular mass compressing the IVC (Fig. 3). These two imaging studies, with tumor marker findings, strongly suggested diagnosis of HCC. Positron emission tomography showed an 8 cm-sized heterogeneously hypermetabolic mass (maximal standardized uptake value [SUV]=4.0) (Fig. 4), which was not regarded as being a hypermetabolic uptake.
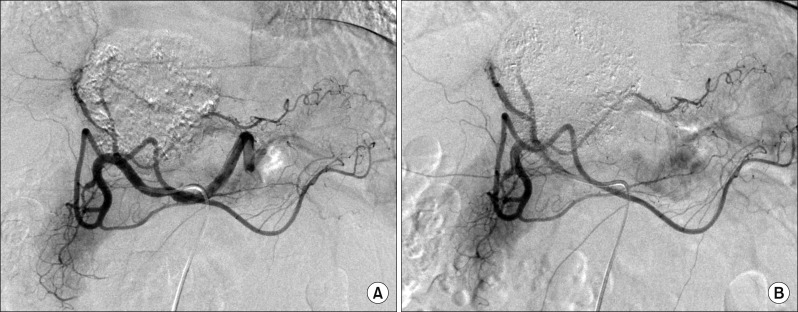
Since this mass was directly attached to the IVC, thus there was a high risk of tumor cell spread through the short hepatic veins, which can induce early pulmonary metastasis. For fear of such a catastrophic pulmonary metastasis, we decided to perform sequential TACE-radiotherapy instead of upfront hepatectomy, although the tumor appeared resectable. The tumor was primarily fed by the left hepatic artery and conventional TACE was performed using chemoinfusion of cisplatin and embolization with Lipiodol and Gelfoam (Fig. 5).
Four days later, a liver CT scan was taken, in which some suspected viable tumor portion was found in the lipiodolized HCC (Fig. 6). We decided to carry out the pre-planned sequential TACE-radiotherapy. A CT simulation was taken 3 weeks after the first session of TACE (Fig. 7). SBRT of 30 Gy with 12 fractions for 2 week was performed in order to avoid irradiation-associated bleeding from the gastric antrum.
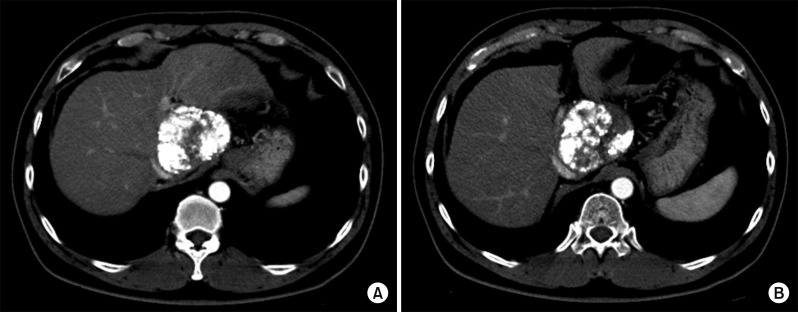
Six weeks later, after SBRT, a follow-up CT scan showed a slight decrease in the size of the lipiodol-uptake HCC, in which we could not exclude the possibility of a viable tumor (Fig. 8). At this time, the serum PIVKA-II level was normalized. Thus, the second session of TACE was performed, in which suspicious tumor staining was found in both lobes, thus chemoinfusion and lipiodol/gelfoam embolization was performed via the right and left hepatic arteries (Fig. 9).
Follow-up CT scans taken every 3 months for 5 years showed no changes of the partial lipiodolized lesion at the caudate lobe without definite arterial enhancement (Fig. 10). A Chest CT scan was taken concurrently, twice per year, showing no evidence of pulmonary metastasis. After finishing follow-up of the first 5 years with no evidence of HCC recurrence, the follow-up interval at the outpatient clinic was extended to 4 months.
The patient showed normal blood glucose levels, but metformin 500 mg has been prescribed twice per day since the second session of TACE for the purpose of chemoprevention.
HCC located at the caudate lobe with encircling involvement of the retrohepatic IVC has often been treated with HR through various surgical approach methods.3456 The transhepatic approach, dividing the hepatic parenchyma to expose the paracaval portion, is a feasible method used to reduce the risk of tumor handling-associated tumor spread. Downstaging with preoperative TACE had been attempted, but its therapeutic effect was worse than expected.711 HR, used only a few months after TACE, should be avoided because the integrity of the HCC cells is loosened, by which the risk of intraoperative tumor spread increases. The preoperative waiting period after TACE is usually recommended to be at least 3-6 months. So far, it is not recommended to perform any preoperative locoregional treatment of resectable or marginally resectable HCCs located at the caudate lobe.
Considering that both HR and TACE for use with HCC at the caudate lobe often resulted in inferior outcomes compared with those at other liver portions, as well as radiofrequency ablation (RFA), is usually not applicable for HCCs at the caudate lobe; it is not simple to choose the treatment modality for patients with HCC at the caudate lobe. HCC with portal vein invasion has been attempted with a combination of TACE and radiotherapy, which resulted in improved outcomes.1213 This therapeutic approach encouraged us to perform a combination of TACE and radiotherapy for HCC at the caudate lobe with a curative intent.
Our protocol for a combination of TACE and radiotherapy with a curative intent in patients with marginally resectable HCC, including HCC at the caudate lobe, includes conventional TACE once or twice in 1-month intervals and then subsequent SBRT within 1 month after the last TACE. Follow-up protocol includes monthly dynamic liver CT scans twice, bimonthly CT scans twice and then routine CT scan follow-up every 3 months. A blood tumor marker study is performed during every visit to the outpatient clinic. A chest CT scan is performed concurrently with every other liver CT scan. During follow-up, if tumor recurrence is detected, we perform repeat TACE, RFA, cryoablation, or even HR as indicated. We think that only hypervascular HCC is indicated for a combination of TACE and radiotherapy. If vascularity of the HCC is not definitely increased, we prefer performing upfront HR without performing any locoregional treatment in the case of HCC with marginal resectability.
Recently, the therapeutic role of radiotherapy for HCC has been emphasized more than before. The local control rate of SBRT is reported to be similar to that of RFA,89 which suggests the possibility of SBRT as an ablative therapy for small HCC. Specifically, respiratory-gated volumetric-modulated arc therapy can shorten the treatment delivery time when compared with SBRT using static beams. Although the clinical impact of a prolonged treatment delivery time on SBRT at a high dose per fraction is not yet clear, from a biological perspective, a prolonged delivery time has shown a detrimental effect on tumor control by reducing cell killing in cell lines or in xenograft models of tumors with a low alpha/beta ratio.141516
The clinical sequence of our present case appears to be unique. The mass at the caudate lobe grows very slowly, indicating malignant transformation within the dysplastic nodule during follow-up over several years. At the time of admission, this lesion was diagnosed of overt HCC. During 5-year follow-up after TACE-radiotherapy, it regressed slowly but probably the non-tumorous portion remained. These sequences are not typical, thus not being presented in detail in literature.
In our present case, metformin has been administered for chemoprevention although he was not diabetic. Metformin is a biguanide agent used to treat type 2 diabetes mellitus. It regulates the blood sugar by improving insulin sensitivity and reducing hepatic glucose output through inhibition of gluconeogenesis and glycogenolysis. Recently, metformin has proven capable of inhibiting cancer cell growth by inducing cell cycle arrest and enhancing apoptosis.17 A considerable number of studies have found that metformin plays a chemopreventive role in other cancers and is associated with reduced risk for HCC.1819 We recently presented that the administration of metformin showed a tendency to reduce the tumor recurrence rate and helped induce significant improvement in overall survival in patients who underwent HR for HCC.20 We suggest administering metformin in HCC patients with glucose intolerance or overt diabetes mellitus.
In conclusion, we presented the clinical sequence of a patient showing complete response of HCC at the caudate lobe after a combination of TACE and radiotherapy. This type of locoregional treatment can be a therapeutic option for HCC at the caudate lobe with marginal resectability.
References
1. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379:1245–1255. PMID: 22353262.

3. Wang ZG, Lau W, Fu SY, Liu H, Pan ZY, Yang Y, et al. Anterior hepatic parenchymal transection for complete caudate lobectomy to treat liver cancer situated in or involving the paracaval portion of the caudate lobe. J Gastrointest Surg. 2015; 19:880–886. PMID: 25759077.

4. Ahanatha Pillai S, Sathyanesan J, Perumal S, Ulagendra Perumal S, Lakshmanan A, Ramaswami S, et al. Isolated caudate lobe resection: technical challenges. Ann Gastroenterol. 2013; 26:150–155. PMID: 24714918.
5. Liu P, Qiu BA, Bai G, Bai HW, Xia NX, Yang YX, et al. Choice of approach for hepatectomy for hepatocellular carcinoma located in the caudate lobe: isolated or combined lobectomy? World J Gastroenterol. 2012; 18:3904–3909. PMID: 22876044.

6. Chaib E, Ribeiro MA Jr, Souza YE, D'Albuquerque LA. Anterior hepatic transection for caudate lobectomy. Clinics (Sao Paulo). 2009; 64:1121–1125. PMID: 19936187.

7. Ha TY, Hwang S, Lee YJ, Kim KH, Ko GY, Ii Gwon D, et al. Absence of benefit of transcatheter arterial chemoembolization (TACE) in patients with resectable solitary hepatocellular carcinoma. World J Surg. 2016; 40:1200–1210. PMID: 26666422.

8. Jeong Y, Jung J, Cho B, Kwak J, Jeong C, Kim JH, et al. Stereotactic body radiation therapy using a respiratory-gated volumetric-modulated arc therapy technique for small hepatocellular carcinoma. BMC Cancer. 2018; 18:416. PMID: 29653562.

9. Ohri N, Dawson LA, Krishnan S, Seong J, Cheng JC, Sarin SK, et al. Radiotherapy for hepatocellular carcinoma: new indications and directions for future study. J Natl Cancer Inst. 2016; 108:djw133. PMID: 27377923.

10. Rim CH, Seong J. Application of radiotherapy for hepatocellular carcinoma in current clinical practice guidelines. Radiat Oncol J. 2016; 34:160–167. PMID: 27730805.

11. Kang WH, Hwang S, Song GW, Lee YJ, Kim KH, Ahn CS, et al. Prognostic effect of transarterial chemoembolization-induced complete pathological response in patients undergoing liver resection and transplantation for hepatocellular carcinoma. Liver Transpl. 2017; 23:781–790. PMID: 28240808.

12. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018; DOI: 10.1001/jamaoncol.2017.5847. [in press].
13. Im JH, Yoon SM, Park HC, Kim JH, Yu JI, Kim TH, et al. Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumour thrombosis in a hepatitis B endemic area. Liver Int. 2017; 37:90–100.

14. Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. stereotactic radiotherapy for liver cancer. BMC Cancer. 2010; 10:475. PMID: 20813065.

15. Wang JZ, Li XA, D'Souza WD, Stewart RD. Impact of prolonged fraction delivery times on tumor control: a note of caution for intensity-modulated radiation therapy (IMRT). Int J Radiat Oncol Biol Phys. 2003; 57:543–552. PMID: 12957268.

16. Zheng XK, Chen LH, Yan X, Wang HM. Impact of prolonged fraction dose-delivery time modeling intensity-modulated radiation therapy on hepatocellular carcinoma cell killing. World J Gastroenterol. 2005; 11:1452–1456. PMID: 15770720.

17. Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011; 71:4366–4372. PMID: 21540236.

18. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013; 62:606–615. PMID: 22773548.

19. Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010; 30:750–758. PMID: 20331505.

20. Kang WH, Tak E, Hwang S, Song GW, Jwa E, Lee YJ, et al. Metformin-associated chemopreventive effects on recurrence after hepatic resection of hepatocellular carcinoma: from in vitro to a clinical study. Anticancer Res. 2018; 38:2399–2407. PMID: 29599368.

Fig. 1
Computed tomography (CT) findings of the liver mass (A) at the caudate lobe taken 3 years before admission. This mass compresses the retrohepatic inferior vena cava (B).
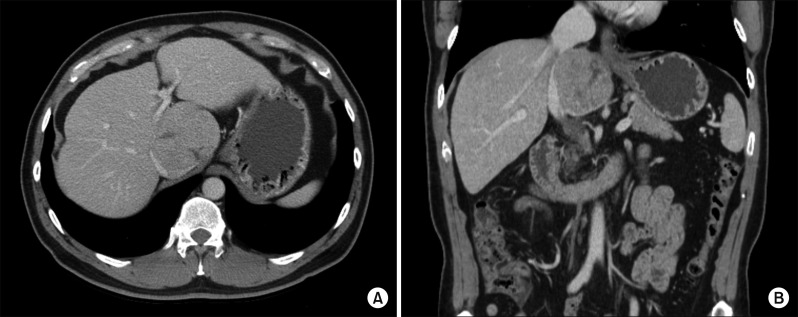
Fig. 2
CT findings of the liver mass at the caudate lobe at admission with pre-enhancement phase (A), arterial phase (B), portal phase (C) and delayed phase (D).
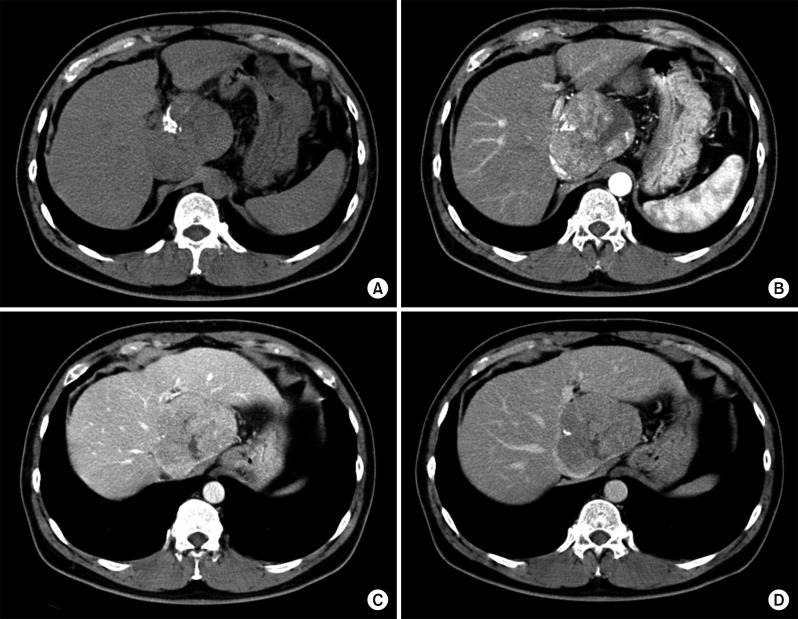
Fig. 3
Magnetic resonance imaging showing a hypervascular mass (A) compressing the inferior vena cava (B).
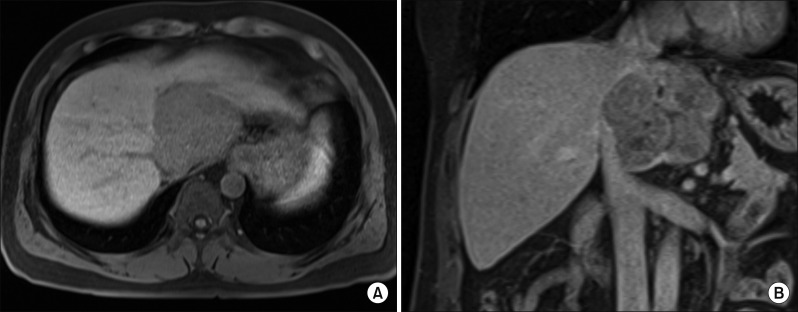
Fig. 5
Hepatic arteriography shows the feeding arteries from the left hepatic artery (A) and post-embolization status (B).
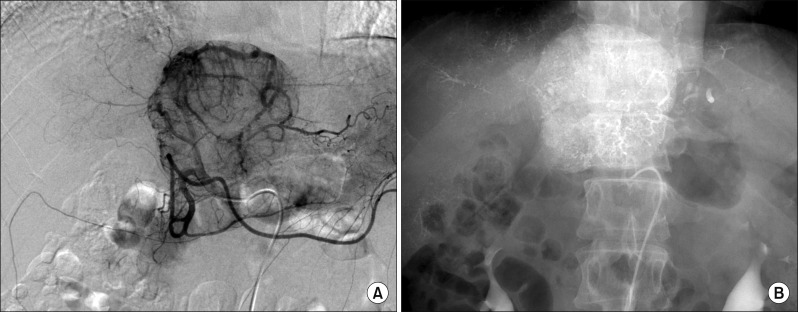
Fig. 6
Liver CT scan of pre-enhancement (A) and arterial phase (B) taken 4 days after transcatheter arterial chemoembolization (TACE) shows a lipiodolized mass.
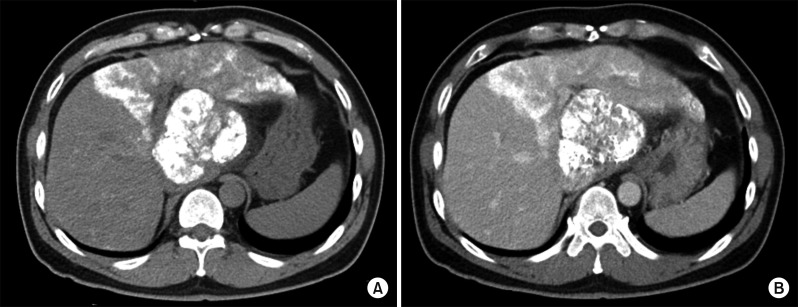
Fig. 7
CT simulation for stereotactic beam radiotherapy with avoidance of exposure to the gastric antrum.
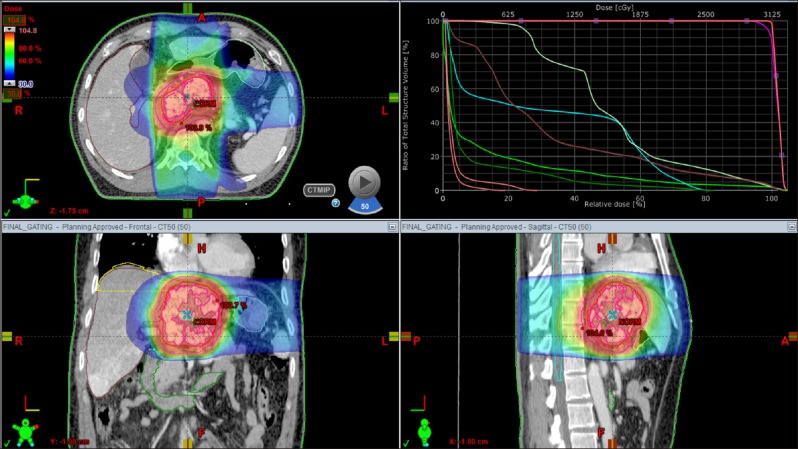
Fig. 8
Liver CT scan of arterial phase (A, B) shows suspected viable tumor portion in the lipiodolized mass.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download