Abstract
A 51-year-old man developed anterograde amnesia following the ingestion of glufosinate ammonium. Brain MRI revealed hyperintense lesions involving the bilateral hippocampus and parahippocampal gyrus, and the right occipital lobe. The mechanism underlying acute glufosinate ammonium intoxication and the differential diagnosis of hippocampal lesions are discussed.
Glufosinate ammonium is a commercial herbicide made from a phosphinic acid derivative of glutamate, which leads to a lethal accumulation of ammonia in plants by inhibiting the activity of glutamine synthetase (1). As the use of glufosinate-ammoniumcontaining herbicides has increased, so too has the incidence of acute intoxication through accidental or purposeful (attempted suicide) ingestion of this herbicide ingestion. The prompt and accurate diagnosis of glufosinate ammonium intoxication is critical from the management perspective.
Acute glufosinate ammonium intoxication is associated with various neurological symptoms including loss of consciousness, convulsion, and memory impairment (1, 2, 3). New memory formation occurs in the hippocampus, and lesions in the bilateral hippocampus result in an inability to form new memories, a condition known as anterograde amnesia (4, 5).
This report describes the case of a patient who presented with anterograde amnesia following the ingestion of a glufosinate-ammonium-containing herbicide in an attempted suicide. The anterograde amnesia was related to a lesion in the hippocampus. The differential diagnosis of hippocampal lesions is discussed.
A 51-year-old man was transferred to an emergencycare center in a stuporous mental state. According to his wife, a bottle of glufosinate ammonium herbicide was present nearby the patient when he was found in this state. Gastric lavage was performed at a neighborhood community hospital, and a nasogastric tube was placed in situ. The doctors made the reasonable assumption that the patient had drunk the herbicide in an attempt to commit suicide, but the amount of glufosinate ammonium ingested was not known. His initial vital signs, including blood pressure and pulse rate, were stable. A baseline chest X-ray and ECG revealed no abnormality. This patient had visited a psychiatry clinic for depressive mood 3 years previously, and had received a percutaneous coronary intervention for acute myocardial infarction 6 months before the poisoning event.
Several hours after his presentation at the hospital, the patient's spontaneous respiration deteriorated, and he underwent endotracheal intubation and ventilator therapy. Chest CT revealed airspace consolidation and volume loss in the dependent portion of the lung, suggesting aspiration pneumonia with atelectasis.
He recovered consciousness on the third day after his admission, but exhibited general disability of expression and comprehension. He was unable to remember things he had just been told, but he could recall events of decades ago; he did not understand why he had been admitted to hospital, despite being told the reason repeatedly by his caregiver. He could not remember whether he had eaten or not, or recall the person who had recently visited him. He was disoriented as to his geographical location and the date. His inability to retain memory and cognitive dysfunction were evident on formal neuropsychological testing performed 2 weeks after symptom onset. The patient scored 18/30 on the Korean version of the Mini Mental State Examination (K-MMSE), 2/5 on the Clinical Dementia Rating (CDR) scale, and 6/7 on the Global Deterioration Scale (GDS).
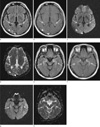
An MRI scan (1.5-T Signa Excite, GE Healthcare, Milwaukee, WI, USA) was performed 10-days after the symptom onset. Fluid-attenuated inversion recovery and T2-weighted images revealed increased signal intensities in the bilateral hippocampus and parahippocampal gyrus, and in the right occipital lobe (Fig. 1). Diffusion-weighted MRI (DWI) also revealed subtle hyperintense lesions in those areas. The regions of interest were drawn in the lesions of the hippocampus and parahippocampal gyrus to allow calculation of the intensity of the lesions on DWI and the apparent diffusion coefficient (ADC) value (Table 1). ADC values (mean and SD) were calculated from the values in five areas (parahippocampal gyrus, hippocampus head, body, and tail, and putamen for reference). The ADCs ranged from 912.5×10-6 to 1242.2×10-6 mm2/s, while the reference value from the putamen (without signal alteration) was 688.1-693.1×10-6 mm2/s. Parenchymal enhancement was observed on gadolinium-enhanced T1-weighted imaging.
Follow-up MRI performed 3 months later revealed subtle hyperintensity and slight atrophy of the hippocampus, and follow-up neuropsychological tests performed 4 months after symptom onset revealed a slightly improved K-MMSE score of 23/30. There were no changes in the other parameters, including CDR and GDS scores, during the follow-up period.
The patient described herein developed anterograde amnesia after acute glufosinate ammonium poisoning, with high signal intensities in the bilateral hippocampus and parahippocampal gyrus, and in the right occipital lobe on brain MRI. Among the spectral neurological symptoms associated with acute glufosinate ammonium poisoning, this patient initially presented with stuporous mentality and subsequent amnesia after recovery of consciousness, similar to the case report by Park et al. (2). On the other hand, Lee et al. were unable to confirm the presence of memory impairment due to the patient's death one week after the acute poisoning (3). Another report presented the case of a patient with glufosinate ammonium poisoning who suffered convulsions and sixth-nerve palsy, without memory impairment or MRI lesions (6). The patient in the present study exhibited modest but selective memory dysfunctions on neuropsychiatry tests, with relative sparing of the other neurological functions.
To the best of our knowledge, this is only the second case to reveal bilateral hippocampal damage in relation to memory impairment after acute glufosinate ammonium poisoning (2), despite it being widely known that the hippocampus plays a crucial role in learning, memory, and emotional behavior (4, 5). Therefore, this study might be a good example case to show that bilateral hippocampal lesions are associated with short-term memory processing. Previous studies have only observed white-matter hyperintensities (1) and a reversible splenial lesion in a patient with retrograde amnesia (7). Although it has a different etiology, the relationship between a brain lesion and memory deficits is similar to that shown in a clinical study of acute heat stroke (5).
It has been suggested that the N-methyl-D-aspartate (NMDA)-type glutamate receptor is the target of glufosinate ammonium in the CNS, although this remains to be confirmed unequivocally (8, 9, 10). Glufosinate acts as a structural analog of the neurotransmitter glutamate, which is recycled within astroglia. Through excessive stimulation of NMDA receptors, glufosinate ammonium increases the production of nitric oxide, thus causing CNS injury. It can be inhibited by NMDA-type glutamate receptor antagonists, but not by other types of receptor antagonist. Although there are currently no animal models for studying the mechanism underlying memory impairment in acute toxicity, memory impairments related to acute glufosinate ammonium intoxication may be indirectly hypothesized from chronic models involving small or moderate doses of the poison (11, 12).
The neurons in the hippocampus function via glutamate receptors, and glutamate is the main neurotransmitter in this brain region. Therefore, hippocampal neurons appear to be susceptible to excitotoxicity via NMDA receptors (2, 3, 11, 12). Although physiological activation of these receptors is necessary for cell survival, as mentioned above, excessive stimulation of NMDA receptors acts as a signal for cell death. In a small-animal model, hippocampal structures were modified and hippocampal glutamine synthetase activity significantly increased in response to chronic glufosinate ammonium exposure (11, 12).
The hippocampus can be affected in several distinct neurological diseases, thus differential diagnosis is required to exclude other etiologies such as acute stroke, global or focal hypoxia, transient global amnesia (TGA), epilepsy, infections, and other metabolic encephalopathies (4). Acute ischemic stroke in the posterior cerebral artery (PCA) territory can be a common mimicker, since the infarct patterns of the PCA territory correspond well with the vascular anatomy of the hippocampus. Additional cerebral lesions supplied by the PCA-such as the thalamus, the splenium, or the occipital lobe-can almost always be found in hippocampal stroke, and isolated hippocampal stroke is a rare finding. Therefore, a PCA stroke was ruled out from the differential diagnosis in the present case. The possibility of TGA was also excluded because the patient did not exhibit complete functional recovery after 24-48 hours, and those of seizure-related parenchymal changes and limbic encephalitis were excluded due to absence of seizures.
This study has the limitation of being a single case report with a retrospective design. The absence of acute-phase DWI made it difficult to differentiate vasogenic edema from cytotoxic edema, but based on the clinical history the lesions were regarded as subacute, dating back to the time of MRI. The DWI and ADC map revealed hyperintensities, and gadolinium-enhanced T1-weighted imaging revealed parenchymal enhancement, suggesting disruption of the blood-brain barrier. The lesion evolved into a chronic phase with a slight volume loss at 3-month follow-up MRI.
Notwithstanding that the amount of poison ingested was uncertain, the important clue in the present diagnosis was the likely history of acute glufosinate ammonium intoxication. Without this information the diagnosis may have been more difficult, such as differentiating from other encephalopathies induced by various agents.
In summary, a bilateral hippocampal lesion presenting as anterograde amnesia may provide diagnostic clues, and acute glufosinate ammonium intoxication can be considered one of the differential diagnoses in the presence of a recent history of drug ingestion.
Figures and Tables
Fig. 1
Brain MRI of a 51-year-old man on the 10th day following glufosinate ammonium intoxication (a-d, basal ganglia level; e-h, midbrain level). (a) Fluidattenuated inversion recovery (FLAIR) image and (b) gadolinium-enhanced T1-weighted imaging (Gd-T1WI) at the basal ganglia level showing bilateral hyperintensities and parenchymal enhancement in the bilateral hippocampus (arrows) and right occipital lobe (arrowheads). This lesion exhibited hyperintensities on (c) diffusion-weighted imaging (DWI) and (d) apparent diffusion coefficient (ADC) mapping, suggesting vasogenic edema. The same pattern was observed in the bilateral hippocampus and parahippocampal gyrus (arrows) on (e) FLAIR, (f) Gd-T1WI, (g) DWI, and (h) ADC mapping at the midbrain level.
Acknowledgements
This work was supported by a grant from the Research Institute of Medical Science, Catholic University of Daegu that was awarded in 2014. The authors have no conflicts of interest to disclose.
References
1. Watanabe T, Sano T. Neurological effects of glufosinate poisoning with a brief review. Hum Exp Toxicol. 1998; 17:35–39.
2. Park HY, Lee PH, Shin DH, Kim GW. Anterograde amnesia with hippocampal lesions following glufosinate intoxication. Neurology. 2006; 67:914–915.
3. Lee HY, Song SY, Lee SH, Lee SY, Kim SH, Ryu SW. Vasogenic edema in striatum following ingestion of glufosinate-containing herbicide. J Clin Neurosci. 2009; 16:1372–1373.
4. Förster A, Griebe M, Gass A, Kern R, Hennerici MG, Szabo K. Diffusion-weighted imaging for the differential diagnosis of disorders affecting the hippocampus. Cerebrovasc Dis. 2012; 33:104–115.
5. Cha SY, Kang TH, Kim SJ, et al. Selective anterograde amnesia associated with hippocampal and splenial damage after heat stroke. Clin Neurol Neurosurg. 2013; 115:1867–1870.
6. Park JS, Kwak SJ, Gil HW, Kim SY, Hong SY. Glufosinate herbicide intoxication causing unconsciousness, convulsion, and 6th cranial nerve palsy. J Korean Med Sci. 2013; 28:1687–1689.
7. Kim TY, Park DW, Park CK, Lee YJ, Lee SR. Reversible splenial lesion in the corpus callosum on MRI after ingestion of a herbicide containing glufosinate ammonium: a case report. J Korean Soc Radiol. 2014; 70:399–402.
8. Nakaki T, Mishima A, Suzuki E, Shintani F, Fujii T. Glufosinate ammonium stimulates nitric oxide production through N-methyl D-aspartate receptors in rat cerebellum. Neurosci Lett. 2000; 290:209–212.
9. Matsumura N, Takeuchi C, Hishikawa K, Fujii T, Nakaki T. Glufosinate ammonium induces convulsion through N-methyl-D-aspartate receptors in mice. Neurosci Lett. 2001; 304:123–125.
10. Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005; 11:37–49.
11. Calas AG, Richard O, Même S, et al. Chronic exposure to glufosinate-ammonium induces spatial memory impairments, hippocampal MRI modifications and glutamine synthetase activation in mice. Neurotoxicology. 2008; 29:740–774.
12. Meme S, Calas AG, Montécot C, et al. MRI characterization of structural mouse brain changes in response to chronic exposure to the glufosinate ammonium herbicide. Toxicol Sci. 2009; 111:321–330.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download