Abstract
Lymphoplasmacyte-rich meningioma is a rare WHO Grade I subtype of meningioma. The lymphoplasmacyte-rich meningioma does not have typical imaging features of a meningioma so it can mimic intracranial inflammatory condition or brain neoplasm. We report the clinicopathologic features of lymphoplasmacyte-rich meningioma in a 35-year-old woman. She suffered from progressive headache, dizziness and tinnitus over two years. The tumor exhibited atypical neuroimaging features, including obvious peritumoral edema and irregular enhancing components. She underwent total resection and histologic examination revealed a meningioma with numerous plasma cells. Her symptoms have since resolved and there has been no evidence of tumor recurrence after one year of follow-up.
Meningiomas are mostly benign tumors that comprise approximately 13% - 26% of all intracranial tumors (1). Lymphoplasmacyte-rich meningioma is a subtype adopted by the new World Health Organization Classification of Tumors of the Central Nervous System and is characterized by the presence of ordinary meningeal components with a background of massive plasma cell and lymphocytic infiltrate (2).
Lymphoplasmacyte-rich meningiomas are uncommon and few reports emphasizing corresponding imaging findings have been published (3). The pathophysiology of this tumor is unknown. The lymphoplasmacyte-rich meningioma does not have typical imaging features of a meningioma, and it is therefore difficult to differentiate with intracranial inflammatory and malignant brain tumor (4). We describe the radiologic and pathologic findings of a new case of lymphoplasmacyte-rich meningioma.
A 35-year-old woman presented with intermittent dizziness, tinnitus and headache lasting two years with projectile vomiting over the past several days. Her past medical and family histories were nonspecific.
Neurologic examination was normal, and a complete blood count (CBC), erythrocyte sedimentation rate (ESR), liver and kidney chemistries, coagulation profile and urinalysis were within normal limits.
The study was approved by our institutional review board and the requirement for informed consent was waived for this case report.
Computed tomography revealed a mixed mass with a nodular solid area, internal fluid-fluid level and marked surrounding edema in left parietal lobe, resulting in mid-line shift and subfalcine herniation to contralateral side (Fig. 1).
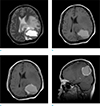
Brain magnetic resonance imaging revealed an irregular and heterogeneously enhancing solid area, intratumoral cystic changes with a hemorrhagic fluid-fluid level, broad basement on the dura and heavy peritumoral brain edema. The enhancing portion exhibited hypointense to isointense signal on T1-weighted sequences and hypointense signal on T2-weighted sequences. There was no destruction of bony structure (Fig. 2). These features appeared to be atypical for a meningioma. As such, the preoperative imaging diagnosis included neoplasms, such as atypical meningioma, metastatic tumor, gliosarcoma, solitary fibrous tumor and inflammatory pseudotumor, sinus histiocytosis.
Neuronavigation-guided craniotomy was performed with gross total resection. On gross examination, the tumor showed cystic changes with partially solid area.
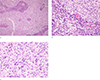
On pathologic examination, the tumor was found to be of the meningothelial subtype of meningioma with lobules that had indistinct borders and positive immunostaining for epithelial membrane antigen (EMA). Numerous plasma cells with Russell bodies were present in the perivascular region and inside the lobules, indicating that it was a lymphoplasmacyte-rich meningioma. The tumor cells showed a few nuclear pseudoinclusions and infrequent mitotic figures. Necrosis, small cell formation, prominent nucleoli, and brain parenchymal invasion were not identified. The Ki-67 proliferative index was 2% (Fig. 3).
The postoperative course was uneventful and the patient's symptoms resolved. Follow-up MRI confirmed total resection of the tumor. There was no evidence of tumor recurrence over one year of follow-up.
There are two clinical features of lymphoplasmacyte-rich meningioma. The first is a predilection for a younger age group (mean of 40.7 years), whereas peak occurrence of typical meningioma is in sixth and seventh decades (4). The other feature is a slightly lower female predominance (male to female ratio 1:1.4) when compared with other meningiomas (1:1.9) (5).
Radiologically, lymphoplasmacyte-rich meningiomas usually show unclear boundary, obvious edema and invasion of adjacent brain tissue (3). These features suggest a high degree of malignancy, but histological examination revealed that these features were due to extensive inflammatory cell infiltration rather than tumor cell invasion (4). Lee et al. (6) suggested that the peritumoral brain edema is probably related to the amount of infiltrate within the tumor, blood supply, and pathological type. Cystic components can also be encountered as in our case (4). The imaging findings of lymphoplasmacyte-rich meningioma are different from other types of common meningioma, thus the differential diagnosis may be extensive, ranging from neoplastic to reactive disorders.
Various hypotheses have been proposed to explain the infiltration of lymphoplasmacytes. The question arises as to whether the lesion is indeed primarily neoplastic or granulomatous change with a secondary meningeal reaction. Banerjee and Blakwood regarded lymphoplasmacyte-rich meningioma as a collision tumor with plasmacytoma components and ordinary meningiomas (7). Bruno et al. (8) suggests that the lymphoplasmacyte-rich meningioma can be considered intracranial inflammatory masses rather than neoplasms due to their biological behavior, immunoprofile and clinical course. The proportion of inflammatory and meningothelial components vary considerably among reported cases (9). Currently, it is not possible to determine whether the interspersed meningothelial cells are reactive or neoplastic, or whether they are primary or secondary to inflammation.
According to previous reports, lymphoplasmacyte-rich meningiomas have a favorable prognosis with little recurrence (10). Our patient did not undergo postoperative radiotherapy, and there has been no evidence of tumor recurrence over one year of follow-up.
In conclusion, we have described a histologically proven lymphoplasmacyte-rich meningioma, mimicking malignancy. The preoperative differential diagnosis is broad, ranging from neoplastic to reactive disorders. However, the recognition of this unusual lymphoplasmacyte-rich meningioma may prompt radiologists to consider it among the differential diagnoses of brain tumors with aggressive features.
Figures and Tables
Fig. 1
Non-enhanced CT demonstrates a cystic tumor mass with an internal fluid-fluid level and extensive brain edema, resulting in mid-line shift.
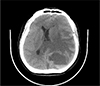
Fig. 2
The lesion demonstrates cystic component with hemorrhagic fluid-fluid level and extensive edematous change on axial T2-weighted image (a) and axial T1-weighted image (b). Irregular thick enhancing wall is noted on contrast-enhanced T1-weighted axial image (c). The mass shows broad dural based attachment on contrast-enhanced T1-weighted sagittal image (d).
References
1. Marosi C, Hassler M, Roessler K, et al. Meningioma. Crit Rev Oncol Hematol. 2008; 67:153–171.
2. Louis DN, Ohgaki H, Weistler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.
3. Liu Jl, Zhou Jl, Ma YH, Dong C. An analysis of the magnetic resonance imaging and pathology of intracal lymphoplasmacyte-rich meningioma. Eur J Radiol. 2012; 81:968–973.
4. Zhu HD, Xie Q, Gong Y, et al. Lymphoplasmacyte-rich meningioma: our experience with 19 cases and a systematic literature reviews. Int J Clin Exp Med. 2013; 6:504–515.
5. Yamaki T, Ikeda T, Sakamoto Y, Ohtaki M, Hashi K. Lymphoplasmacyte-rich meningioma with clinical resemblance to inflammatory pseudotumor. Report two cases. J Neurosurg. 1997; 86:898–904.
6. Lee KJ, Joo WI, Rha HK, et al. Peritumoral brain edema in meningiomas: correlations between magnetic resonance imaging, angiography, and pathology. Surg Neurol. 2008; 69:350–355.
7. Banerjee AK, Blackwood W. A subfrontal tumor with the features of plasmocytoma and meningioma. Acta Neuropathol. 1971; 18:84–88.
8. Bruno MC, Ginguené C, Santangelo M, et al. Lymphoplasacyte rich meningioma. A case report and review of the literature. J Neurosurg Sci. 2004; 48:117–124.
9. Horten BC, Urich H, Stefoski D. Meningiomas with conspicuous plasma cell-lymphocystic components: a reportion of five cases. Cancer. 1979; 43:258–264.
10. Loh JK, Hwang SL, Tsai KB, Kwan AL, Howng SL. Sphenoid ridge lymphoplasmacyte-rich meningioma. J Formos Med Assoc. 2006; 105:594–598.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download