Abstract
Cauda equina syndrome (CES) is often defined as a complex of symptoms and signs consisting of low back pain, bilateral sciatica, lower extremity weakness, saddle anesthesia, and bowel and bladder dysfunction. CES is considered to be neurosurgical emergency. Delayed or missed diagnosis of CES can result in serious morbidity and neurological sequelae. However, the diagnosis of CES is often difficult when one or more of these symptoms are absent or when these symptoms develop asymmetrically or incompletely. We report a case of urinary retention and sphincter dysfunction without sciatica or motor weakness following an L3 burst fracture in a 52-year-old male and discuss the atypical presentation of CES and treatment of traumatic CES.
Cauda equina syndrome (CES) is a complex of clinical symptoms, including low back pain (LBP), bilateral leg pain, weakness in the lower extremities, saddle anesthesia, genitourinary dysfunction with overflow incontinence or retention, sexual dysfunction, and loss of rectal sphincter tone, occasionally with fecal incontinence.45) The diagnosis of CES is not difficult in patients with all of these symptoms. However, the diagnosis is difficult when these symptoms develop asymmetrically or incompletely or one or more of these symptoms are absent. Even if the initial presentation is incomplete or atypical, CES can progress and potentially cause severe neurological symptoms and disability.5) Patients with CES should be referred immediately for surgical consideration, as treatment delay may result in serious morbidity, such as loss of bladder, bowel, and sexual function with potential legal consequences.9) Therefore, it is important that all clinicians who assess patients with LBP should have a detailed knowledge of the atypical presentation and signs highly suggestive of CES. A few cases of atypical presentation of CES due to lumbar disc herniation have been reported, but atypical presentation of CES following a lumbar spinal fracture have been rarely reported. We report a case of urinary retention and sphincter dysfunction without sciatica or motor weakness following a L3 burst fracture in a 52-year-old male and discuss the atypical presentation of CES and treatment of traumatic CES.
A 52-year-old man presented to the emergency department with LBP. The patient fell from approximately 2 m-container box to the ground 2 hours previously. He complained of no pain and no numbness in either leg. A neurological examination revealed grade 5/5 muscle strength and no sensory changes in the lower extremity. Normal rectal tone was present and there was no saddle anesthesia. Plain lateral radiography and computed tomography (CT) scan showed a burst fracture with spinous process fracture at L3 with more than 50% of the vertebral body height loss measured on Picture Archiving and Communication System (π-ViewSTAR, INFINITT Healthcare Co., Seoul, Korea) (Figure 1A, B, and C). Spinal canal diameter was estimated at the level of the fracture on the axial CT image that traversing the level of the pedicle and was defined as the distance between the midsagittal border of the posteriorly dislocated bone fragment and the lamina.20) The normal size of the canal was estimated by the average midsagittal diameter of the levels adjacent to the fracture.20) Spinal canal diameter at the fractured level was 7.92 mm using this method and about 55% narrowing of the spinal canal was observed compared to the normal level (Figure 1C). Magnetic resonance imaging (MRI) revealed findings suspicious of injury in the supraspinous ligament (Figure 1D). Initially, we thought the patient had no neurological deficits, so internal fixation and posterior fusion without decompression of the thecal sac were planned under the impression of an unstable burst fracture.26)
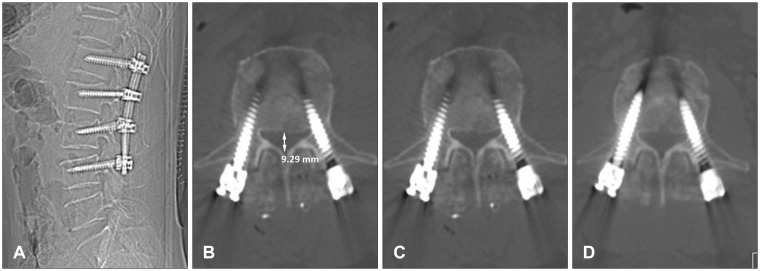
He underwent pedicle screw fixation from L1 to L4 and posterior fusion the next day, with internal distraction to reduce the fragment away from the spinal canal. Motor strength and sense in his legs were intact postoperatively. Pain was tolerable 3 days postoperatively, and the patient began to ambulate without difficulty. The Foley catheter was removed. He did not feel the desire to urinate. He was able to expel only a few drops of urine by applying pressure to the anterior abdominal wall when he tried to void at the time of 500 cc of urine in his bladder measured with bladder ultrasonography. This was managed initially by regular intermittent catheterization, but he complained of urethral discomfort. A Foley catheter was placed again the next day. He also complained of constipation, but this improved with kinetic agents (mosapride 10 mg three times a day) and laxatives (magnesium oxide 500 mg three times a day). Plain lateral radiography and CT scan confirmed restoration of sagittal alignment and appropriate positioning of the screws (Figure 2A and B). Although spinal canal diameter increased slightly to 9.29 mm, significant thecal sac compression was detected (Figure 2C). We recommend MRI and additional anterior decompressive surgery, but he refused because of uncertainity of recovering from voiding difficulty after the surgery and his poor economic status. The Foley catheter was removed again 1 week later, but he did not void as before. Electromyography (EMG), nerve conduction study (NCS), and urodynamic study (UDS) were performed 3 weeks after the surgery. EMG and NCS showed no abnormalities in L2-S1 roots. However, abnormal spontaneous activity and decreased motor action potential amplitude in the abductor hallucis muscle were detected, which did not exclude the possibility of S2 root denervation. Further electrodiagnostic evaluation for pudendal somatosensory evoked potentials and anal sphincter EMG were recommended, but he refused. No bladder detrusor muscle activity and areflexic neurogenic bladder were found in the UDS. He was managed with suprapubic cystostomy for 3 months. Voiding improved gradually during that time. CT scan of the lumbar spine at that time revealed a more resolved fractured bone fragment, and the spinal canal diameter increased to 10.94 mm (Figure 2D). After removing the cystostomy, he was able to void by himself, but had a weak stream and residual urine. He voided with abdominal straining or sitting on the toilet seat to void fully. Voiding has not returned to normal at the 15-month follow-up.
A prompt and correct diagnosis of CES is sometimes difficult. When symptoms or signs of CES develop asymmetrically or incompletely or one or more of symptoms are absent, the diagnosis is difficult. Cases with atypical presentation of CES, such as unilateral sciatica, without sciatica or motor weakness, without saddle anesthesia, with only fecal incontinence and perineal hypesthesia, and without bladder involvement, have been reported.59171922) CES presenting with only urinary dysfunction without sciatica and motor weakness, as in the present case, in patients with centrally protruded L4-5 lumbar disc herniation has also been reported.24) The cauda equina is arranged with the higher nerve roots travelling more laterally, and the lower sacral nerve roots travelling within the medial aspect of the cauda.4) The S2-5 roots at the L2-3 and L3-4 level are located most medially near the midline within the spinal canal.23) Therefore, a large compressive midline lesion can only affect the sacral nerve roots, which forms the pelvic splanchnic nerves arising from the S2-4 roots. Pelvic splanchnic nerves control the bladder detrusor muscle which contracts during micturition to aid in emptying the bladder.4) In the present case, a fractured bone fragment located near the midline at the L3 level may have compressed the S2-5 nerve roots without affecting the lumbar nerve roots.
In addition, there are marked inconsistencies in the current evidence base surrounding the clinical presentation of CES.9) Fraser et al.9) reported that defining CES is problematic because 17 different definitions had been proposed in the literatures. Definitive symptoms or signs for diagnosing CES vary among authors. Balasubramanian et al.1) suggested that saddle sensory deficit has a higher predictive value than other clinical features when diagnosing CES and a degree of canal compromise greater than 75% is capable of producing CES. Bell et al.2) recommended an urgent MRI assessment in all patients who present with new onset urinary symptoms in the context of LBP or sciatica. Domen et al.7) reported that urinary retention above 500 mL after micturition measured by bladder scan is the most promising diagnostic tool to predict the presence of cauda equina compression on MRI. Gooding et al.11) argued that a digital rectal examination has no significant value in the diagnosis of CES and cannot be used as a discriminator to rationalize an urgent MRI. Fairbank et al.8) noted that there is limited evidence from individual symptoms or signs from a patient's history or clinical examination, respectively, can be used to diagnose CES in their systematic review.
To overcome these discrepancies, Fraser et al.9) proposed a single definition of CES after reviewing 105 articles and, one or more of the following must be present for a diagnosis of CES: 1) bladder and/or bowel dysfunction, 2) reduced sensation in the saddle area, or 3) sexual dysfunction, with possible neurological deficits in the lower limb (motor/sensory loss or reflex change). Based on the above mentioned proposal, the presence of mild saddle anesthesia or urinary retention are important findings for diagnosing CES, particularly when patients have no leg pain and when no motor weakness or sensory changes are found on a neurological examination. However, these findings can often be missed in a trauma patient or during the postoperative period.1012) Routine placement of a Foley catheter during the initial resuscitation after trauma or during the postoperative period can make urinary dysfunction difficult to detect.1012) In the present case, an indwelling Foley catheter was used in the emergency department after initial neurological examination and radiologic evaluation of plain film and CT. But the volume of urine drained immediately after inserting the catheter, which could be the clue for voiding difficulty, was not checked because no abnormal finding suggestive of CES, such as weakness or saddle anesthesia, was found in the neurological examination. The patient had Foley catheter for 3 days after the surgery. Therefore, it was too late to detect the voiding difficulty and this may have made the voiding difficulty long-lasting or perhaps permanent.
The effect of surgical treatment on recovery of neurological deficits in patients with a thoracolumbar or lumbar spinal fracture remains controversial. Some authors have found no association between initial canal encroachment, final spinal canal area, the extent of decompression, treatment technique, or spinal level of injury and neurological recovery.6131621) Although nonsurgical care of traumatic CES likely results in some neurological improvement, many authors advocate that the vast majority of these injuries should be treated with surgical stabilization and, when necessary, concomitant decompression. Not only is this likely to reduce the duration of hospital stay and facilitate nursing and rehabilitation, but it is clearly safe from a neurological perspective and may optimize neurological recovery.18) Hu et al.14) evaluated 69 patients with lumbar fractures and incomplete neurological deficits and noted that patients had statistically greater motor improvement, regardless of whether the decompression was anterior or posterior, compared with patients who underwent posterior fusion alone at a mean 19 month follow-up. Kaneda et al.15) retrospectively reviewed 69 patients with traumatic CES. Nearly 75% of patients had complete neurological recovery and complete recovery was found in 9 of 12 patients with voiding difficulty after anterior decompression and stabilization.15) Bradford and McBride3) retrospectively examined data from 59 patients with thoracic and lumbar fractures and 17 had CES. There were highly significant rates of bowel and bladder recovery between the posteriorly (11.7%) and anteriorly (70%) treated groups. The timing of surgery in patients with traumatic CES also remains controversial. Thongtrangan et al.25) reported that 14 of 17 patients had satisfactory outcomes and recommended that surgery be performed within 48 hours of CES onset. Although controversies remains, early decompressive surgery can increase the chance of recovery from neurological deficits including bladder or bowel dysfunction.31415) However, in the present case, missing CES during the early period of trauma led us to fixation and fusion of fractured site without decompression of the nerve roots, and this may have reduced the chances of recovering bladder dysfunction.
Symptoms of CES may be atypical. Suspicion of bladder or bowel dysfunction is very important particularly when no motor or sensory changes are evident. A high index of suspicion of CES is essential when encountering patients with LBP or a history of trauma to the lumbar spine to prevent missing a diagnosis of CES and avoiding severe neurological symptoms and disabilities.
References
1. Balasubramanian K, Kalsi P, Greenough CG, Kuskoor Seetharam MP. Reliability of clinical assessment in diagnosing cauda equina syndrome. Br J Neurosurg. 2010; 24:383–386. PMID: 20726746.

2. Bell DA, Collie D, Statham PF. Cauda equina syndrome: what is the correlation between clinical assessment and MRI scanning? Br J Neurosurg. 2007; 21:201–203. PMID: 17453789.
3. Bradford DS, McBride GG. Surgical management of thoracolumbar spine fractures with incomplete neurologic deficits. Clin Orthop Relat Res. 1987; (218):201–216. PMID: 3568482.

4. Caputo LA, Cusimano MD. Atypical presentation of cauda equina syndrome. J Can Chiropr Assoc. 2002; 46:31–38.
5. Celik EC, Kabatas S, Karatas M. Atypical presentation of cauda equina syndrome secondary to lumbar disc herniation. J Back Musculoskelet Rehabil. 2012; 25:1–3. PMID: 22398260.

6. Dai LY, Wang XY, Jiang LS. Neurologic recovery from thoracolumbar burst fractures: is it predicted by the amount of initial canal encroachment and kyphotic deformity? Surg Neurol. 2007; 67:232–237. discussion 238PMID: 17320624.

7. Domen PM, Hofman PA, van Santbrink H, Weber WE. Predictive value of clinical characteristics in patients with suspected cauda equina syndrome. Eur J Neurol. 2009; 16:416–419. PMID: 19490073.

8. Fairbank J, Hashimoto R, Dailey A, Patel AA, Dettori JR. Does patient history and physical examination predict MRI proven cauda equina syndrome? Evid Based Spine Care J. 2011; 2:27–33. PMID: 23230403.

9. Fraser S, Roberts L, Murphy E. Cauda equina syndrome: a literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009; 90:1964–1968. PMID: 19887225.

10. Gitelman A, Hishmeh S, Morelli BN, Joseph SA Jr, Casden A, Kuflik P, et al. Cauda equina syndrome: a comprehensive review. Am J Orthop (Belle Mead NJ). 2008; 37:556–562. PMID: 19104682.
11. Gooding BW, Higgins MA, Calthorpe DA. Does rectal examination have any value in the clinical diagnosis of cauda equina syndrome? Br J Neurosurg. 2013; 27:156–159. PMID: 23113877.

12. Harrop JS, Hunt GE Jr, Vaccaro AR. Conus medullaris and cauda equina syndrome as a result of traumatic injuries: management principles. Neurosurg Focus. 2004; 16:e4. PMID: 15202874.

13. Herndon WA, Galloway D. Neurologic return versus cross-sectional canal area in incomplete thoracolumbar spinal cord injuries. J Trauma. 1988; 28:680–683. PMID: 3367414.
14. Hu SS, Capen DA, Rimoldi RL, Zigler JE. The effect of surgical decompression on neurologic outcome after lumbar fractures. Clin Orthop Relat Res. 1993; (288):166–173. PMID: 8458130.

15. Kaneda K, Taneichi H, Abumi K, Hashimoto T, Satoh S, Fujiya M. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg Am. 1997; 79:69–83. PMID: 9010188.
16. Kim NH, Lee HM, Chun IM. Neurologic injury and recovery in patients with burst fracture of the thoracolumbar spine. Spine (Phila Pa 1976). 1999; 24:290–293. discussion 294PMID: 10025025.

17. Kim TW, Yoon JW, Heo W, Park HS, Rhee DY. Lumbar Disc Herniation Presenting Cauda Equina Syndrome. J Korean Neurosurg Soc. 2006; 39:40–45.
18. Kingwell SP, Curt A, Dvorak MF. Factors affecting neurological outcome in traumatic conus medullaris and cauda equina injuries. Neurosurg Focus. 2008; 25:E7. PMID: 18980481.

19. Kostuik JP, Harrington I, Alexander D, Rand W, Evans D. Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg Am. 1986; 68:386–391. PMID: 2936744.

20. Meves R, Avanzi O. Correlation between neurological deficit and spinal canal compromise in 198 patients with thoracolumbar and lumbar fractures. Spine (Phila Pa 1976). 2005; 30:787–791. PMID: 15803082.

21. Mohanty SP, Venkatram N. Does neurological recovery in thoracolumbar and lumbar burst fractures depend on the extent of canal compromise? Spinal Cord. 2002; 40:295–299. PMID: 12037711.

22. O'Laoire SA, Crockard HA, Thomas DG. Prognosis for sphincter recovery after operation for cauda equina compression owing to lumbar disc prolapse. Br Med J (Clin Res Ed). 1981; 282:1852–1854.
23. Orendácová J, Cízková D, Kafka J, Lukácová N, Marsala M, Sulla I, et al. Cauda equina syndrome. Prog Neurobiol. 2001; 64:613–637. PMID: 11311464.
24. Sylvester PA, McLoughlin J, Sibley GN, Dorman PJ, Kabala J, Ormerod IE. Neuropathic urinary retention in the absence of neurological signs. Postgrad Med J. 1995; 71:747–748. PMID: 8552542.

25. Thongtrangan I, Le H, Park J, Kim DH. Cauda equina syndrome in patients with low lumbar fractures. Neurosurg Focus. 2004; 16:e6. PMID: 15202876.
FIGURE 1
Lumbar spine images immediately after trauma. Plain lateral radiography (A) and sagittal (B) view of the computed tomography scan show the L3 burst fracture and about 50% height loss in the vertebral body. Midsagittal spinal canal diameter estimated at the level of the fracture in the transpedicular axial cut is 7.92 mm, which represents 55% of narrowing of the spinal canal compared to the normal level. Spinous process fracture is also seen (white arrow) (C). Fat-suppressed T2-weighted magnetic resonance imaging reveals suspicious supraspinous ligament injury (black arrow) (D).
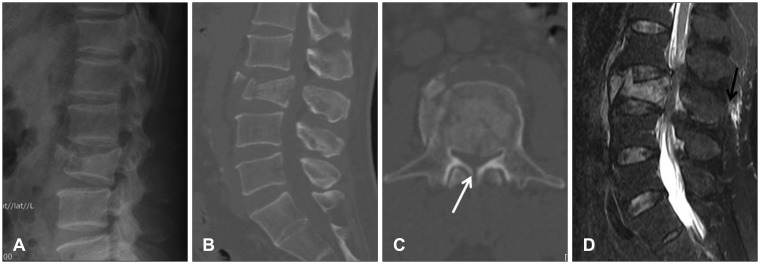
FIGURE 2
Postoperative lumbar spine images. Plain lateral radiography (A) and sagittal (B) view of the computed tomography scan show restoration of spinal alignment and slight reduction of the retropulsed bony fragment. Midsagittal spinal canal diameter is 9.29 mm, which was slightly increased compared to the preoperative images (C). Midsagittal spinal canal diameter is 10.94 mm 3 months after the surgery (D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download