Abstract
Objective
Structural adaptation of the vascular wall may occur due to various factors, such as shear stress, pressure, injury or inflammation. The role of microRNAs (miRNAs) in the development of vascular remodeling has been investigated in several studies. Recently, the authors reported altered expression profiles of miRNAs in late stage of experimentally induced giant cerebral aneurysm (CA) in rat models. But, early biologic roles of miRNAs in CA formation have not been explained yet. We employed microarrays analysis to identify miRNA expression profiles in early stage of CA in rat model and to compare with those in late stage of giant CA.
Methods
Seventy, 7-week-old male Sprague-Dawley rats underwent a CA induction procedure. The control animals (n=11) were fed a regular diet, and the experimental animals (n=59) were fed a regular diet with 1% normal saline for two months. Then, the rats were killed, their cerebral arteries were dissected, and the 13 regions of early aneurysmal change on the right olfactory artery-anterior cerebral artery bifurcation were cut for miRNA microarrays analysis. Six miRNAs (miRNA-1, miRNA-448, miRNA-352, miRNA-551b, miRNA-431, and miRNA-485) were randomly chosen for validation using real-time quantitative polymerase chain reaction.
Results
Among a set of differentially expressed miRNAs, 15 miRNAs were up-regulated more than 200% and five miR-NAs were down-regulated less than 50% in the early CA tissues.
Conclusions
This study provides an overall view of miRNA expression profiles in experimentally induced early CAs and strongly supports the idea that some miRNAs, such as miR-31 and miR-27a, play an important role in pathological processes in early CA formation. Further investigations to detect their exact roles of these miRNAs in the pathogenesis of CA are needed.
References
1. Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol. 30:1118–1126. 2010.

2. Aoki T, Nishimura M, Kataoka H, Ishibashi R, Nozaki K, Miyamoto S. Complementary inhibition of cerebral aneurysm formation by eNOS and nNOS. Lab Invest. 91:619–626. 2011.

3. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
4. Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, et al. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J. 29:559–573. 2010.
5. Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 49:2379–2393. 2007.
6. Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 105:158–166. 2009.

7. Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 460:705–710. 2009.

8. Fazi F, Nervi C. MicroRNA: basic mechanisms and transcriptional regulatory networks for cell fate determination. Cardiovasc Res. 79:553–561. 2008.

9. Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 100:1579–1588. 2007.

10. Lee HJ, Yi JS, Lee HJ, Lee IW, Park KC, Yang JH. Dysregulated Expression Profiles of MicroRNAs of Experimentally Induced Cerebral Aneurysms in Rats. J Korean Neurosurg Soc. 53:72–76. 2013.

11. Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 106:15819–15824. 2009.

12. Liu X, Cheng Y, Chen X, Yang J, Xu L, Zhang C. MicroRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2. J Biol Chem. 286:42371–42380. 2011.

13. Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 104:476–487. 2009.

14. Neth P, Nazari-Jahantigh M, Schober A, Weber C. MicroRNAs in flow-dependent vascular remodelling. Cardiovasc Res. 99:294–303. 2013.

15. Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 464:1196–1200. 2010.

16. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 84:767–801. 2004.

18. Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 469:336–342. 2011.

19. Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 15:261–271. 2008.

20. Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 393:643–648. 2010.

FIGURE 1.
Two months after the induction procedures. The aneurysm samples of different stages were obtained from the right olfactory artery-anterior cerebral artery (OA-ACA) bifurcation area. A: Normal OA-ACA bifurcation. B: Early cerebral aneurysm at OA-ACA bifurcation (black arrowhead). C: Advanced cerebral aneurysm at OA-ACA bifurcation (black arrowhead). ACA: anterior cerebral artery, OA: olfactory artery (×400 magnification).
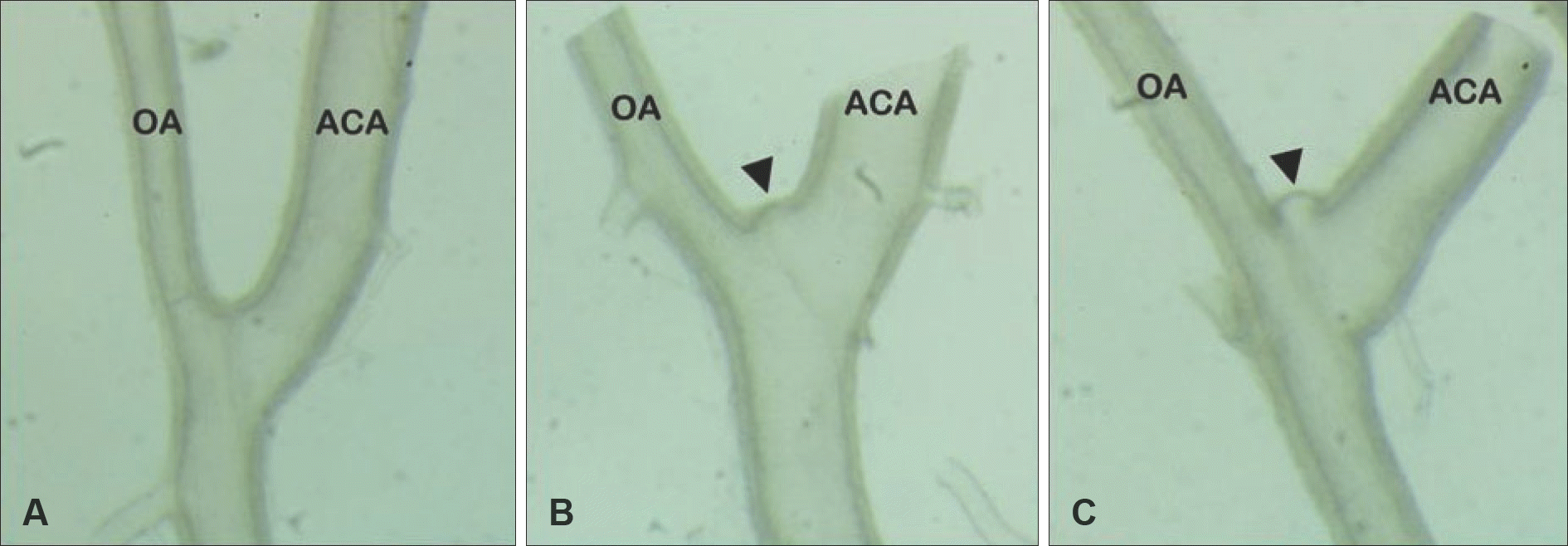
FIGURE 2.
Hierarchical clustering analysis of miRNA expression of early cerebral aneurysms and control cerebral arteries. miRNAs are presented in rows and samples are presented in columns. Colors indicate relative signal intensities; red and blue colors indicate up-expressed and down-expressed miRNAs, respectively.
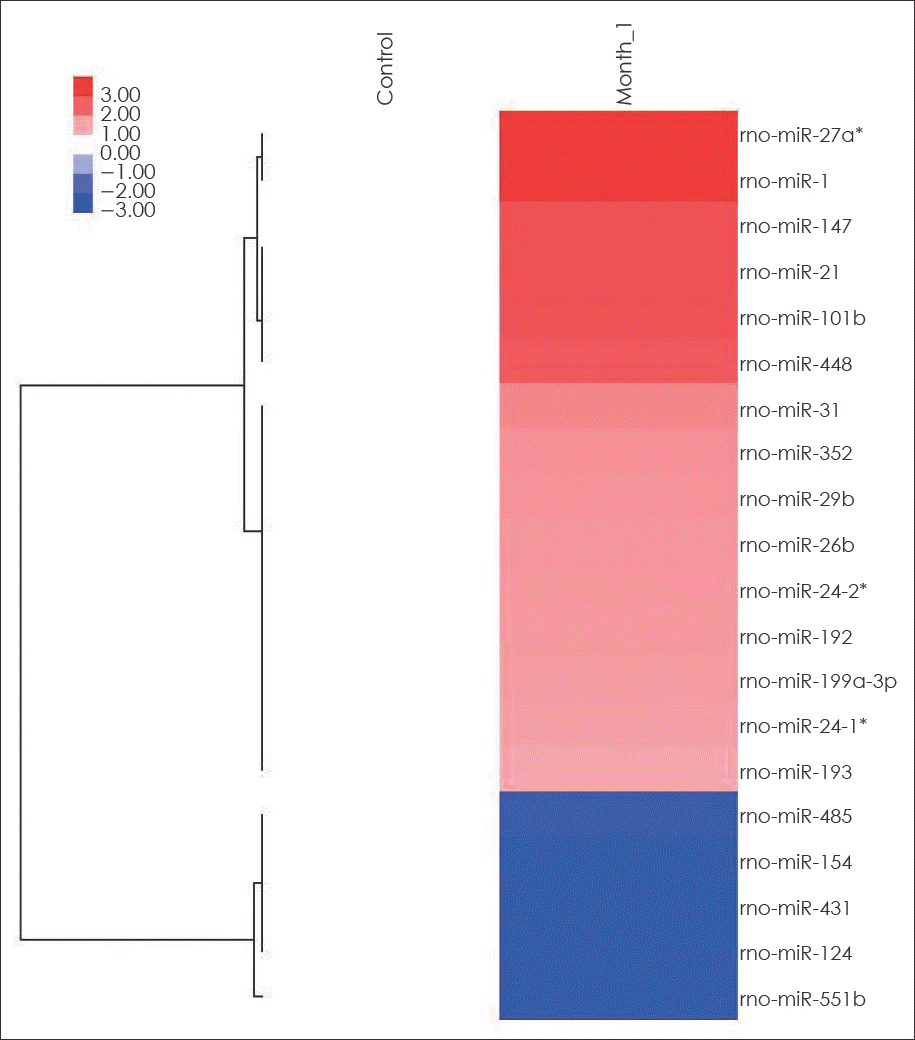
FIGURE 3.
Summary of real-time quantitative polymerase chain reaction (qPCR) analysis for the up-expressed miRNAs (miRNA-1, miRNA-448, and miRNA-352) and the down-expressed miRNAs (miRNA-551b, miRNA-431, and miRNA-485). Six randomly selected miRNAs are listed on the x-axis, and relative expression levels are placed on the y-axis as up and down direction. ∗significant difference between CAs and control arteries (p<0.05). CA: cerebral aneurysm.
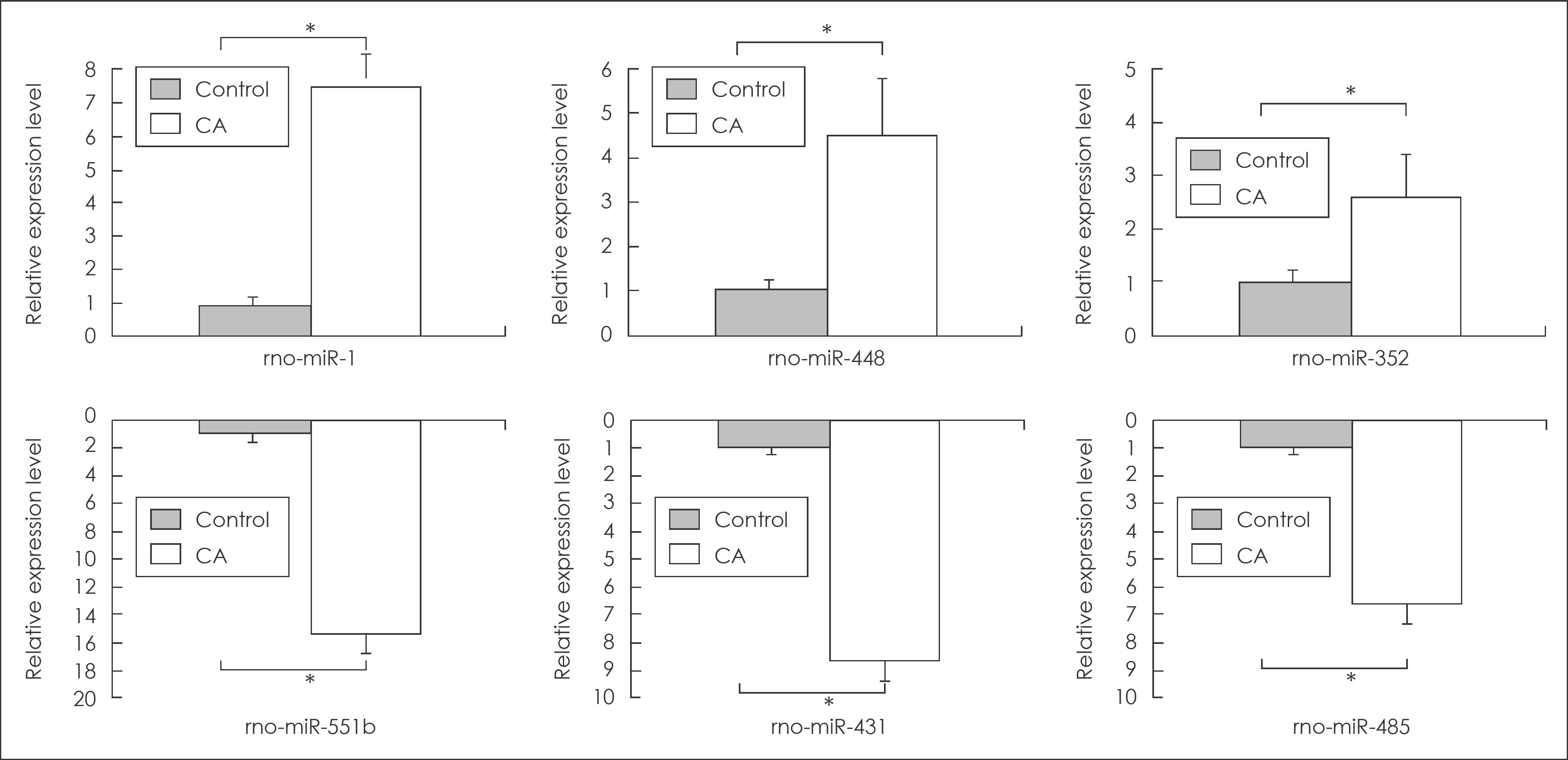
TABLE 1.
The list of altered microRNA expression in the earl cerebral aneurysm tissue




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download