International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) E17 is a guideline for general principles for Planning and Design of Multi-Regional Clinical Trials (MRCTs). The preparation process started in June 2014 and draft was issued in June 2016. The guideline was finalized in November 2017 and is now being implemented by member countries (step 5).[1]
MRCT is defined in the present guideline as a clinical trial conducted in more than one region under a single protocol. In this context, a region may refer to a geographical region, country or regulatory region. The primary focus of this guideline is on MRCTs designed to provide data that will be submitted to multiple regulatory authorities for drug approval (including approval of additional indications, new formulations and new dosing regimens) and for studies conducted to satisfy post-marketing requirements. A successful MRCTs will enable earlier access to innovative therapies by synchronizing clinical drug development across different regions, avoid duplication and reduce the need for region specific studies and bridging studies. It will also provide better evidences for drug approval in each region by incorporation of latest knowledge and experience from regions into one trial.[2]
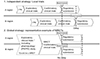
The intrinsic and extrinsic factors important to the drug development program, should be identified so that the potential impact of these factors could be examined in the exploratory phases before the design of confirmatory MRCTs. MRCTs are planned under the assumption that the treatment effect applies to the entire target population, particularly to the regions included in the trial. Pooling in this guideline means integrating a subset of the subjects from one particular region with those from similarly defined other regions. Pre-specified pooling is important for strategic allocation of the sample size to regions for global development. For this pooling, it is important to execute well-planned early development programs that include PK and/or PK-PD studies of applicable parameters, in order to identify regional differences which may impact dose selection in confirmatory MRCTs (Fig. 1).[2]
The early phase exploratory MRCTs can gather scientific data regarding the impact of extrinsic and intrinsic factors on PK, PK/PD and/or other drug properties. Those early phase MRCTs may also serve as the basis for approval in regions not studied at the confirmatory stage through the extrapolation of study results.
East Asian regions including Korea, Japan and China are good candidate for pooling as these regions have the advantages due to the following similarities that are discrete from the Western countries: race, genotype of several drug metabolizing enzymes, phenotype, and disease prevalence. Nevertheless, diversities exist within the regions that require evaluation of ethnic sensitivities. But most of the global drug development programs have not included early phase clinical pharmacology trials in this region except for some bridging studies or ethnic PK studies for Japanese. Therefore, various efforts by several East Asian co-operative groups such as KoNECT[3] and regulatory bodies have been involved in promotion and capacity building for exploratory trials in this region. Efforts are still under way to identify similarities and differences within the East Asian region in the early phase of drug development, at both governmental and investigators levels. A few past examples of similarity would be the moxifloxacin, simvastatin and meloxicam joint pharmacokinetic (PK) studies by Korea-Japan-China Tripartite co-operation,[4] and investigator initiated trial collaborative researches between Korea and Japan on the atenolol PK.[5]
In the area of adverse drug reactions (ADR), there are reports on difference in prevalence among this Asian and/or Western regions. Docetaxel myelosuppression in Asians are more frequent due to reduced clearance. Doxorubicin myelosuppression in Asians due to PK and carbonyl reductase 3 (CBR3).[6] Gefitinib shows more effect in Asians and marked toxicity in Japanese due to EGFR mutation.[7] Most of these differences were identified in late confirmatory MRCTs or after approval.
Arnold and others observed dose differences in 73 of 190 drugs approved in the US and Japan. They reported that the pathway of drug development is more strongly associated with dose difference than with drug characteristics which includes intrinsic factors.[8] The implication of this report is that finding potential difference in dose by early exploratory trials is more important to confirm the recommended dose difference.
Therefore, regional explorative early phase clinical pharmacology trials are essential when planning confirmatory MRCTs before we accumulate more experience. Clinical pharmacologists in the East Asian region should establish networks for this collaborative early phase MRCTs and provide scientific evidence for similarity or difference in PK, PK-PD, and ADR for successful global drug development.
Figures and Tables
Figure 1
Illustrations of clinical drug development workflow across regions for drug submission and regulatory review in independent and global strategies.[2] *Marketing Authorization Application/New Drug Application, **Could be parallel single region trials or MRCTs.
Notes
References
1. General principles for planning and design of Multi-Regional Clinical Trials. Accessed 1 July 2018. http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/general-principle-on-planningdesigning-multi-regional-clinical-trials.html/.
2. ICH E17. Accessed 1 July 2018. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E17/E17EWG_Step4_2017_1116.pdf/.
3. Construction of Northeast Asian network. Accessed 1 July 2018. https://www.konect.or.kr/kr/contents/business_new_6/view.do/.
4. Hasunuma T, Tohkin M, Kaniwa N, Jang IJ, Yimin C, Kaneko M, et al. Absence of ethnic differences in the pharmacokinetics of moxifloxacin, simvastatin, and meloxicam among three East Asian populations and Caucasians. Br J Clin Pharmacol. 2016; 81:1078–1090. DOI: 10.1111/bcp.12884.

5. Jeon H, Jang IJ, Lee S, Ohashi K, Kotegawa T, Ieiri I, et al. Apple juice greatly reduces systemic exposure to atenolol. Br J Clin Pharmacol. 2013; 75:172–179. DOI: 10.1111/j.1365-2125.2012.04324.x.

6. Hor SY, Lee SC, Wong CI, Lim YW, Lim RC, Wang LZ, et al. PXR, CAR and HNF4alpha genotypes and their association with pharmacokinetics and pharmacodynamics of docetaxel and doxorubicin in Asian patients. Pharmacogenomics J. 2008; 8:139–146.

7. Tamura M, Kondo M, Horio M, Ando M, Saito H, Yamamoto M, et al. Genetic polymorphisms of the adenosine triphosphate-binding cassette transporters (ABCG2, ABCB1) and gefitinib toxicity. Nagoya J Med Sci. 2012; 74:133–140.
8. Arnold FL, Fukunaga S, Kusama M, Matsuki N, Ono S. Assessment of factors associated with dose differences between Japan and the United States. Clin Pharmacol Ther. 2014; 95:542–549. DOI: 10.1038/clpt.2013.231.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download