Abstract
Infectious costochondritis seldom occurs after breast reconstruction. The treatment requires wide debridement, appropriate wound cover, and antibiotic therapy. A 53-year-old female patient was referred due to an unhealed right breast wound. She had undergone right skin-sparing mastectomy followed by breast reconstruction with an implant. Pseudomonas aeruginosa was cultured from the wound discharge, and a computed tomography showed fluid collection underneath the pectoralis muscle with connection to the external opening as well as degenerated T4–T6 costal cartilages. Wide excision of infected tissue and costal cartilages followed by a pedicled latissimus dorsi musculocutaneous flap coverage were performed. The mastectomy wound allows a wider surgical view to prepare thoracodorsal vessels, and the harvesting the latissimus dorsi musculocutaneous flap can be more easily performed without excessive traction force or damage on pedicles. The coverage of pedicled flap was successful and the patient was injected antibiotics intravenously for 3 weeks without any postoperative complications.
Infectious costochondritis is a rare problem after breast reconstruction1. In contrast with inflammatory costochondritis, it can be fatal or chronic, so early diagnosis and appropriate treatment are important. Invariably, the treatment of infectious costochondritis requires surgical exploration, followed by debridement, appropriate wound cover of the anterior chest, and antibiotic therapy2.
Latissimus dorsi musculocutaneous (LDMC) pedicled flap is a reliable and versatile option in reconstruction of the anterior chest3. It can provide large size of flap, incorporating several types of tissue, long pedicle, and robust vessels. The flap can cover a large soft tissue defect with advantages of a large vascularized surface area, long distance migration of flap, and low donor site morbidity45.
In this case, the dissection of the thoracodorsal (TD) vessels were started in the supine position for harvesting an LDMC flap through the anterior wound. It was able to be performed easily to cover an anterior chest wound from infectious costochondritis following silicone implant-based breast reconstruction.
A 53-year-old female patient was referred to our clinical institute for an unhealed right breast wound after right skin-sparing mastectomy for ductal carcinoma in situ followed by breast reconstruction with a permanent implant at another clinic nine months before presentation. Five weeks after the first breast reconstruction at another clinic, the wound had dehisced and demonstrated signs of infection, so the breast implant was removed, and she received intravenous antibiotics for treatment of the infection. However, the wound worsened, and the patient underwent surgical debridement, partial removal of the degenerated costal perichondrium, and serial negative pressure wound therapy.
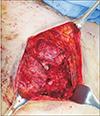
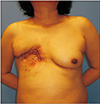
At the time the patient visited our institute, gross examination revealed a 7.0×1.0-cm-sized wound disruption with turbid and foul discharge from the raw wound surface, multiple fistulas, local soft tissue atrophy, and marginal erythema with tenderness (Fig. 1). Pseudomonas aeruginosa was cultured from the wound discharge, and a computed tomography scan showed fluid collection underneath the pectoralis muscle with connecting tracts to the external opening, focally enhanced pleura, and costal cartilage degeneration at T4–T6 (Fig. 2). For treatment, the surgical plan included wide excision of infected tissue and resection of costal cartilages followed by LDMC flap coverage.
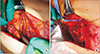
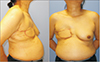
The degenerated pectoralis major muscle was debrided, and 3 tracts to infected costal cartilage were noted, prompting partial resection of the costal cartilage (Fig. 3). After extensive debridement, an approximately 13.0×8.0 cm skin and soft tissue defect from the medial sternum to the right upper breast border was investigated. Elevation of the pedicled LDMC flap commenced, and the TD vessels were explored through the open wound in the supine position. Detaching the perivascular soft tissue with an ultrasound energy device (Ethicon Endo-Surgery, Cincinnati, OH, USA), we dissected the TD vessels under loupe magnification (×3.5) in the following manner. Perivascular detaching was ranged from the bifurcation site with the circumflex scapular artery to the end of branches connecting to the LDMC flap, while the branch to the serratus anterior muscle was ligated (Fig. 4A). The tendon inserted into the LDMC was detached, and the TD nerve was dissected (Fig. 4B). Then the skin flap of the LDMC was elevated in the lateral decubitus position, and the flap was inset in the supine position. After the operation, the patient received intravenous ceftazidime for 3 weeks and was discharged without any postoperative complications (Fig. 5).
Prosthetic inflammation is a common complication after breast reconstruction surgery, but infectious spreading to deep structures like the costal cartilage and rib is rare1. Few infectious costochondritis cases after breast reconstruction or breast augmentation surgery have been reported, and most cases required serial debridement surgery2. However, to the best of our knowledge, there have been no articles reporting infectious costochondritis after breast reconstruction using implant.
Additionally, when costochondritis is suspicious, it is important to differentiate between inflammatory and infective causes. The inflammatory costochondritis is self-limiting and well-healed by the anti-inflammatory medication or steroid infiltration2. On the other hand, the infectious costochondritis like this case requires extensive debridement, resection of multiple costochondral cartilage, and soft tissue reconstruction of anterior chest.
In this case, the breast implant was removed 5 weeks after the first surgery due to wound dehiscence and infection. Two possibilities can be inferred: underestimation of the initial infectious sign and late onset of subclinical infection. Both possible situations would have had infectious signs such as pain, erythema, and heating sensation before the wound dehisced. If infection is suspected, it is necessary to aggressively remove the implant at an early stage and to perform empirical antibiotics treatment for Staphylococcus aureus and P. aeruginosa.
The pedicled LDMC flap is a workhorse flap among the reconstruction options for the anterior chest and breast3. It offers a large size of well-vascularized skin paddle, incorporating several types of tissue, long pedicle, robust vessels, and muscle volume with low donor site morbidity45. Therefore, it is appropriate donor to be selected to treat the chronic and infective wound like this case.
To reconstruct chest wall, general principles are inincluding; stabilizing thoracic skeletal structure altered by respiratory mechanics, obliterating intrathoracic and extrathoracic dead space, and preserving vital intrathoracic structures4. In addition to LDMC flap, pectoralis major flap, rectus abdominis flap, or omental flap are also reported as good candidates.
Traditionally, LDMC flap harvest starts in the lateral decubitus position, and the TD vessels are dissected from the skin paddle456. Various skin incisions and designs of skin islands have been described378, and although there may be differences in degree of difficulty depending on type, TD vessel dissection is performed through a subcutaneous tunnel that extends from skin paddle toward the axilla, and this surgical field is narrow for proper operation.
In the case of this patient, the wide debridement including removal of cartilage from the lesion of costochondritis was performed first in the supine position, so vessel preparation was able to be approached easily before changing position in lateral decubitus. After detaching the tendon inserted into the LDMC, the surgical view becomes much wider than the subcutaneous tunnel. Then the operator can perform sophisticated vessel work in a stable position with atraumatic manipulation of vessels and low rate of damage on pedicles. Because the maximal axis of the TD vessel is long up to 28 cm and the skin paddle is supplied by perforators with a small diameter ranging from 0.5 to 1.5 mm5, then the elaborate manipulation of vessels is essential.
An ultrasound energy device is efficient and safer than the traditional bipolar electrocautery during vessel dissection9. The harmonic scalpel enables the operator to meticulously dissect and perform effective hemostasis with safe ligation of small branches, and its low energy limits the spread of thermal energy to the surrounding tissue. Therefore, this method can decrease surgical time, drainage output, blood loss, and seroma formation compared to the traditional approach10.
In infective costochondritis, some limitations of breast reconstruction with pedicled LDMC flap can be considered. First, after wide debridement, the sufficient volume might not be provided as in this case, therefore it can be used in patients with small breasts or in patients who can embrace asymmetric contours of both breasts. In addition, if the infection invades the axilla, thoracodorsal vessels might not be secure, prudent use of the pedicled LDMC flap will be necessary.
Figures and Tables
Fig. 2
Preoperative computed tomography. (A) A fluid collection underneath the pectoralis muscle (white arrow) with connecting tracts to the external opening (white arrowhead). (B) Degenerated T4–T6 costal cartilages (white arrow).

Fig. 3
Intraoperative photo: the pectoralis major muscle and 3 tracts to infected costal cartilage were resected.
References
1. van Schalkwyk AJ, van Wingerden JJ. A variant of Tietze's syndrome occurring after reconstructive breast surgery. Aesthetic Plast Surg. 1998; 22:430–432.

2. Moses MA, Banwell PE, Murphy JV, Quinlan MJ, Coleman DJ. Infective costochondritis following breast reconstruction. Plast Reconstr Surg. 2004; 114:1356–1357.

3. Chang DW, Youssef A, Cha S, Reece GP. Autologous breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg. 2002; 110:751–759. discussion 760-1.

4. Bakri K, Mardini S, Evans KK, Carlsen BT, Arnold PG. Workhorse flaps in chest wall reconstruction: the pectoralis major, latissimus dorsi, and rectus abdominis flaps. Semin Plast Surg. 2011; 25:43–54.

5. Thomas BP, Geddes CR, Tang M, Williams J, Morris SF. The vascular basis of the thoracodorsal artery perforator flap. Plast Reconstr Surg. 2005; 116:818–822.

6. Saint-Cyr M, Nagarkar P, Schaverien M, Dauwe P, Wong C, Rohrich RJ. The pedicled descending branch musclesparing latissimus dorsi flap for breast reconstruction. Plast Reconstr Surg. 2009; 123:13–24.

7. Aitken ME, Mustoe TA. Why change a good thing? Revisiting the fleur-de-lis reconstruction of the breast. Plast Reconstr Surg. 2002; 109:525–533. discussion 534-8.

8. Hokin JA, Silfverskiold KL. Breast reconstruction without an implant: results and complications using an extended latissimus dorsi flap. Plast Reconstr Surg. 1987; 79:58–66.
9. Lee YJ, Kim HY, Han HH, et al. Comparison of dissection with harmonic scalpel and conventional bipolar electrocautery in deep inferior epigastric perforator flap surgery: A consecutive cohort study. J Plast Reconstr Aesthet Surg. 2017; 70:222–228.

10. Matthews B, Nalysnyk L, Estok R, et al. Ultrasonic and nonultrasonic instrumentation: a systematic review and meta-analysis. Arch Surg. 2008; 143:592–600.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download