Introduction
Sarcoidosis is a systemic disease involving formation of non-caseating granulomas in any organ. In nearly 90% of sarcoidosis patients, a lesion is found in the lung and/or hilar lymph nodes [1]. Sarcoidosis involving the gastrointestinal (GI) tract is rare. It may occur in any region of the GI tract including the esophagus, stomach, small bowel, colon, and appendix, although the lesion occurs most frequently in the stomach [2]. Here, we report our experience with a 60-year-old woman diagnosed with pulmonary sarcoidosis and gastric sarcoidosis accompanied by enlarged abdominal lymph nodes.
Case
A 60-year-old female patient presented to the clinic with abnormal chest radiography. The patient was a homemaker with no smoking history and no significant past medical history including hypertension, hyperlipidemia, and tuberculosis. Vital signs were normal and she was afebrile with no cough, dyspnea, or change in weight. Physical examination showed clear breath sound and no lymphadenopathy.
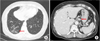
The patient's chest radiograph showed a reticular opacity in the right lower lobe of the lung. Chest computed tomography (CT) revealed interlobular and peribronchovascular septal thickening in the right lower lobe suggestive of idiopathic pulmonary fibrosis, cryptogenic organizing pneumonia, asbestosis, or sarcoidosis (Fig. 1). A 9 mm soft tissue density neighboring the inferior pulmonary vein was also found. Multiple enlarged lymph nodes were observed near the left gastric vessel, which were suspected to be metastatic lymph nodes or lymphangitic lung metastasis from an intra-abdominal malignancy. The patient did not exhibit visual disturbance, nasal obstruction, or skin rash.
The patient's laboratory results were as follows: hemoglobin 12.3 g/dL, white bolld cell (WBC) 5,220/µL, platelet 161,000/µL, C-reactive protein (CRP) 0.12 mg/dL and ionized calcium 5.0 mg/dL. Tumor markers such as carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE) were measured and found to be normal at 0.8 ng/mL and 12.0 ng/mL. Angiotensin converting enzyme (ACE) was elevated at 114 U/L.
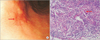
Abdominal CT showed lymphadenopathy along the left gastric artery, splenic artery, gastrocolic trunk, and great omentum, with no masses or thickening in the walls of the stomach (Fig. 1). Esophagogastroduodenoscopy (EGD) revealed a stellate, shallow, whitish discoloration accompanied by mucosal irregularity in the anterior wall of the body and mucosal depression (0.8 cm) along the greater curvature (Fig. 2A). Biopsies of both lesions showed chronic active gastritis with granulomas and multinucleated giant cells containing Schaumann bodies and Sydney Grade 1 Helicobacter pylori. Malignant cells were not observed (Fig. 2B). Colonoscopic findings were normal. Positron emission tomography-computed tomography (PET-CT) showed hypermetabolic lymph nodes in the neck, mediastinum, and abdomen, and diffuse and mild interstitial hypermetabolism in the middle and lower lobe of the right lung, suggesting the possibility of malignant lymphoma or malignancy of unknown origin.
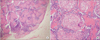
Laparoscopic intra-abdominal lymph node biopsy was performed. It was negative for mycobacterium tuberculosis and non-tuberculous mycobacterium, and showed multiple wellformed, nodular non-necrotizing granulomas, further suggesting a diagnosis of sarcoidosis (Fig. 3). As treatment for suspected pulmonary and gastric sarcoidosis, the patient was prescribed methylprednisolone at 32 mg/day for 14 days followed by a gradual tapering to 16 mg/day over the course of 55 days. Follow-up CT performed after 6 months of treatment showed significant improvement of interlobular septal thickening and decreased size of abdominal lymph nodes.
Discussion
Sarcoidosis is a chronic systemic granulomatous disease of unknown etiology. It may affect any organ and results in noncaseating granulomas [3]. About 90% of patients with sarcoidosis have lesions in the lung and/or hilar nodes and only 0.9~1.0% of the cases involve the GI tract [4].
The most common area of the GI tract affected by sarcoidosis is the stomach and it is usually subclinical in these cases. In 0.1~0.9% of patients, however, clinical manifestations including epigastric pain, nausea, and vomiting are reported [4]. Perigastric lymphadenopathy and gastric ulcers were observed in our case, but other gastrointestinal abnormalities including abdominal pain were not present. EGD of sarcoidosis typically shows a lesion at the antrum with single or multiple ulcerations and mimics the appearance of a peptic ulcer [5]. Other common findings include single or diffuse nodular lesions, a linitis plastica type of lesion with thickening of the gastric wall, "cobblestoning," and cone-shaped antral deformities [6]. In our case, EGD showed mucosal irregularity accompanied by an ulcer scar in the anterior wall of the body and mucosal depression along the greater curvature. These lesions were similar to those reported in cases of gastric sarcoidosis.
Gastric sarcoidosis cases reported thus far involve lesion in the gastrointestinal tract requiring endoscopy, and biopsy reveals non-caseating granulomas. In the majority of cases, the patient was either previously diagnosed with pulmonary sarcoidosis or had bilateral hilar lymphadenopathy, which is typical of sarcoidosis. Recently, a case of a 65-year-old male patient with anorexia, nausea, and watery diarrhea was reported [7]. Despite a normal endoscopy, a biopsy of the gastric fundus revealed non-caseating granulomas. Furthermore, bilateral hilar lymphadenopathy was observed on chest radiography, leading to a diagnosis of pulmonary sarcoidosis. Our case of gastric sarcoidosis is unique in that the abdominal lymphadenopathy typically observed in gastric cancer was present in the setting of reticular opacities typical of pulmonary sarcoidosis that were found on retrospective review of the CT scan.
Sarcoidosis is a diagnosis of exclusion. It should be suspected when non-caseating granulomas are found on biopsy of a lesion. The differential diagnosis should include disease that involve granulomatous inflammation, such as Crohn's disease, foreign body reaction, fungal infection, and tuberculosis [8]. It is important to distinguish sarcoidosis from a sarcoid-like reaction [9]. Sarcoid-like reactions occur via local irritation and therefore tests such as mycobacterium tuberculosis polymerase chain reaction (PCR) or Ziehl-Neelsen staining for tuberculosis [10]; gene studies related to sarcoidosis [11]; and tumor antigen test for lung [12], breast [13], and prostate [14] cancer are needed. In the case reported herein, mycobacterium tuberculosis and non-tuberculosis mycobacterium PCR studies of lymph nodes were all negative. Although tumor antigen testing of lymph nodes was not conducted, malignant cells were not observed and the blood tests for tumor markers were negative, allowing for the exclusion of malignancies such as gastric cancer or lymphoma. Furthermore, the patient had no history of exposure to beryllium, mercury, silica, and asbestos, and there was no observable foreign body. Observation of reticulopathy by chest CT could have been suggestive of idiopathic pulmonary fibrosis or cryptogenic organizing pneumonia, and although transbronchial lung biopsy, bronchoalveolar lavage, and pulmonary function tests had not been previously conducted, no clear respiratory condition existed. This in addition to the results of the stomach and lymph node biopsy, made the possibility of sarcoidosis involving the lung highly likely.
18F-fluorodeoxyglucose (FDG) PET and PET-CT show increased FDG uptake in sarcoidosis. Although this is also true in cases of infection, inflammatory conditions, and malignancy, these methods can be used to determine disease extent and effects of treatment in sarcoidosis [15]. Although in our case no malignant cells were seen in the stomach on endoscopy, there was lymphadenopathy around the stomach, leaving malignancy as a possibility. This led us to conduct a PET-CT, which showed multiple hypermetabolic lymph nodes in the neck, mediastinum, and abdomen, but no lesion in the stomach. Although this observation did not distinguish between sarcoidosis, inflammatory lymphadenopathy, benign lymphoproliferative disease, or metastatic lymph nodes, it helped us determine the best site for surgical biopsy.
In summary, although gastric sarcoidosis is a rare disease, it should be considered as a possibility when non-caseating granulomas are observed in the GI tract in the setting of pulmonary sarcoidosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download