Abstract
Objectives
The aims of this study were to determine the erosive potential of several carbonated waters and to confirm the availability of a simple ISO protocol for screening the erosive potential of drinks.
Methods
A total of six carbonated waters were tested. Three products (Lemon-Sparkling water, Seagram, and Trevi) were domestic, and the other three (Perrier, San Pellegrino, and Rosbacher) were imported. Two kinds of carbonated drinks (Coca-Cola and Sprite) were used as controls. The erosive potential of each drink was assessed by measuring the initial pH (pHI), the final pH after degassing of carbon dioxide (pHF), and the titratable acidity to pH 5.5 (TA5.5) and 7.0 (TA7.0). The pH changes (∆pH) caused by the addition of drinks to screening solutions were calculated according to the ISO protocol for evaluating the erosive potential of oral rinses.
Results
The overall erosive potential of the carbonated waters was lower than that of the control drinks. The pHI and pHF of the carbonated waters ranged from 3.94 to 5.84 and from 5.07 to 7.88, respectively. The Lemon-Sparkling water showed the highest erosive potential among the carbonated waters, having the lowest pH (3.94) and the highest TA5.5 (1.67 ml). The ∆pH of all tested drinks ranged from ―1.00 to 0.23. Also, the tendency of erosive potential measured by ∆pH was similar to that measured by TA5.5.
Conclusions
The carbonated waters tested in this study had a lower erosive potential than did the carbonated drinks. However, the erosive potential of domestic products was higher than that of imported products. The results of the ISO screening test could reflect the influence of the acid content as well as the pH of drinks. Therefore, this protocol could also be conveniently applied to evaluate the erosive potential of various drinks.
References
1. Eccles JD. Dental erosion of nonindustrial origin. a clinical survey and classification. J Prosthet Dent. 1979; 42:649–653.

2. Nunn JH. Prevalence of dental erosion and the implications for oral health. Eur J Oral Sci. 1996; 104:156–161.

3. Jensdottir T, Arnadottir IB, Thorsdottir I, Bardow A, Gudmunds-son K, Theodors A, et al. Relationship between dental erosion, soft drink consumption, and gastroesophageal reflux among Icelanders. Clin Oral Investig. 2004; 8:91–96.

4. Jensdottir T, Bardow A, Holbrook P. Properties and modification of soft drinks in relation to their erosive potential in vitro. J Dent. 2005; 33:569–575.

5. Zero DT. Etiology of dental erosion-extrinsic factors. Euro J Oral Sci. 1996; 104:162–177.
6. Kim BR, Min JH, Kwon HK, Kim BI. Analysis of the erosive effects of children’s beverages using a pH-cycling model. J Korean Acad Oral Health. 2013; 37:141–146.

7. Ehlen LA, Marshall TA, Qian F, Wefel JS, Warren JJ. Acidic beverages increase the risk of in vitro tooth erosion. Nutr Res. 2008; 28:299–303.

8. Salas MM, Nascimento GG, Vargas-Ferreira F, Tarquinio SB, Huys-mans MC, Demarco FF. Diet influenced tooth erosion prevalence in children and adolescents: Results of a meta-analysis and meta-regression. J Dent. 2015; 43:865–875.

9. Parry J, Shaw L, Arnaud MJ, Smith AJ. Investigation of mineral waters and soft drinks in relation to dental erosion. J Oral Rehabil. 2001; 28:766–772.

10. Brown CJ, Smith G, Shaw L, Parry J, Smith AJ. The erosive potential of flavoured sparkling water drinks. Int J Paediatr Dent. 2007; 17:86–91.

11. Ministry of Food and Drug Safety. 2014 Production of food and food additives. Cheongju: Ministry of Food and Drug Safety;2014. p. 125.
12. Edwards M, Creanor SL, Foye RH, Gilmour WH. Buffering capacities of soft drinks: the potential influence on dental erosion. J Oral Rehabil. 1999; 26:923–927.

13. Lussi A, Jaggi T, Scharer S. The influence of different factors on in vitro enamel erosion. Caries Res. 1993; 27:387–393.

14. Lussi A, Megert B, Shellis RP, Wang X. Analysis of the erosive effect of different dietary substances and medications. Br J Nutr. 2012; 107:252–262.

15. Hara AT, Zero DT. Analysis of the erosive potential of calcium-containing acidic beverages. Eur J Oral Sci. 2008; 116:60–65.

16. Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. J Dent Res. 2006; 85:226–230.

17. Schmuck B. Evaluation of three assessment methods: screening for dental erosive capacity. IADR. 2008.
18. International Organization for Standardization. ISO 28888: 2013 Dentistry-screening method for erosion potential of oral rinses on dental hard tissues. Geneva: International Organization for Stan-dardization;2013. p. 1–5.
19. Creanor S, Ferguson J, Foye R. Comparison of the cariogenic potential of caloric and noncaloric carbonated drinks. J Dent Res. 1995; 873–873.
20. Barbour ME, Lussi A. Erosion in relation to nutrition and the environment. Monogr Oral Sci. 2014; 25:143–154.

21. Shellis RP, Barbour ME, Jesani A, Lussi A. Effects of buffering properties and undissociated acid concentration on dissolution of dental enamel in relation to pH and acid type. Caries Res. 2013; 47:601–611.

22. Grenby TH. Lessening dental erosive potential by product modification. Eur J Oral Sci. 1996; 104:221–228.

23. West NX, Hughes JA, Parker DM, Moohan M, Addy M. Development of low erosive carbonated fruit drinks 2. evaluation of an experimental carbonated blackcurrant drink compared to a conventional carbonated drink. J Dent. 2003; 31:361–365.

24. Hughes JA, West NX, Parker DM, Newcombe RG, Addy M. Development and evaluation of a low erosive blackcurrant juice drink in vitro and in situ. 1. Comparison with orange juice. J Dent. 1999; 27:285–289.
25. Larsen MJ, Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999; 33:81–87.

26. Min JH, Kwon HK, Kim BI. The addition of nano-sized hydroxyapatite to a sports drink to inhibit dental erosion-in vitro study using bovine enamel. J Dent. 2011; 39:629–635.

27. Min JH, Kwon HK, Kim BI. Prevention of dental erosion of a sports drink by nano-sized hydroxyapatite in situ study. Int J Paediatr Dent. 2015; 25:61–69.
28. American Dental Association Foundation. ISO collaborative protocol: Evaluation of simplified method to estimate relative erosive potential of oral rinses. Chicago: American Dental Association Foundation;2007. p. 1–4.
Fig. 1.
The results of screening test for erosive capacity of drinks with Ca-PO4 solution. pH, the value after subtraction of initial pH of Ca-PO4 solution from pH of Ca-PO4 solution after addition of drinks. Lemon-S.W: Lemon-Sparkling water; S.pellegrino: San pellegrino. Each test was conducted in quadruplicate with new drinks.
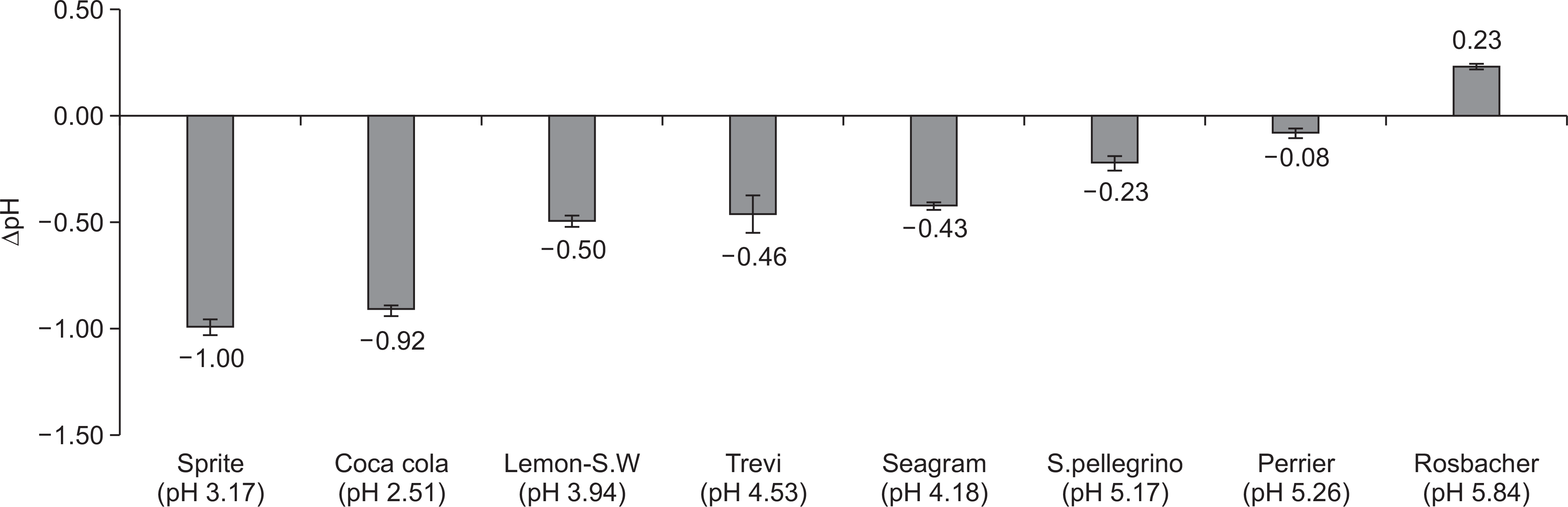
Table 1.
Control drinks (Carbonated drinks) and experimental drinks (carbonated waters) used in this study
| Brand name | Flavour | Manufacturer | Ingredients |
|---|---|---|---|
| Sprite* | Lime, Lemon | Coca-Cola Korea | Purified water, Carbonate dioxide, White sugar, Synthetic favoring substance, |
| Citric acid, Trisodium citrate, Propylene glycol | |||
| Coca-cola* | - | Coca-Cola Korea | Purified water, Carbonate dioxide, High fructose corn syrup, White sugar, caramel, Coloring matter, Phosphoric acid, Natural favoring substance, Caffein |
| Lemon-Sparkling water† | Lemon | CU, Namyang F&B | Purified water, Carbonate dioxide, Natural lemon flavoring |
| Trevi† | Lemon | Lotte Chilsung | Purified water, Carbonate dioxide, Natural lemon flavoring |
| Seagram† | Lemon | Coca-Cola Korea | Purified water, Carbonate dioxide, Natural lemon flavoring |
| Perrier† | Lemon | Nestle Waters supply sud | Purified water, Carbonate dioxide, Natural lemon flavoring |
| San pellegrino† | - | Nestle Waters supply sud | Water, Carbonate dioxide |
| Rosbacher† | Lemon | Hassia Waters international | Purified water, Carbonate dioxide, Natural lemon flavoring |
Table 2.
The pH values of control beverages and experimental carbonated waters used in this study
Table 3.
The amount (ml) of 1 M NaOH required to raise the pH of each drink to 5.5 and 7.0
Table 4.
The rating of erosive potential of drinks in screening test with Ca-PO4 solution and titratable acidity to pH 5.5




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download