Abstract
Background and Objectives
Diet is one of the major risk factors for thyroid diseases. It has been shown that high or excessive iodine intake is more likely to be a health concern in iodine-sufficient regions or regions where iodine deficiency previously existed due to the emergence of iodine-induced hypothyroidism or hyperthy-roidism. Therefore, this review investigates the occurrence of thyroid diseases, and particularly hypothyroidism and hyperthyroidism, in populations with different levels of iodine intake and other dietary factors in various geographic regions.
Materials and Methods
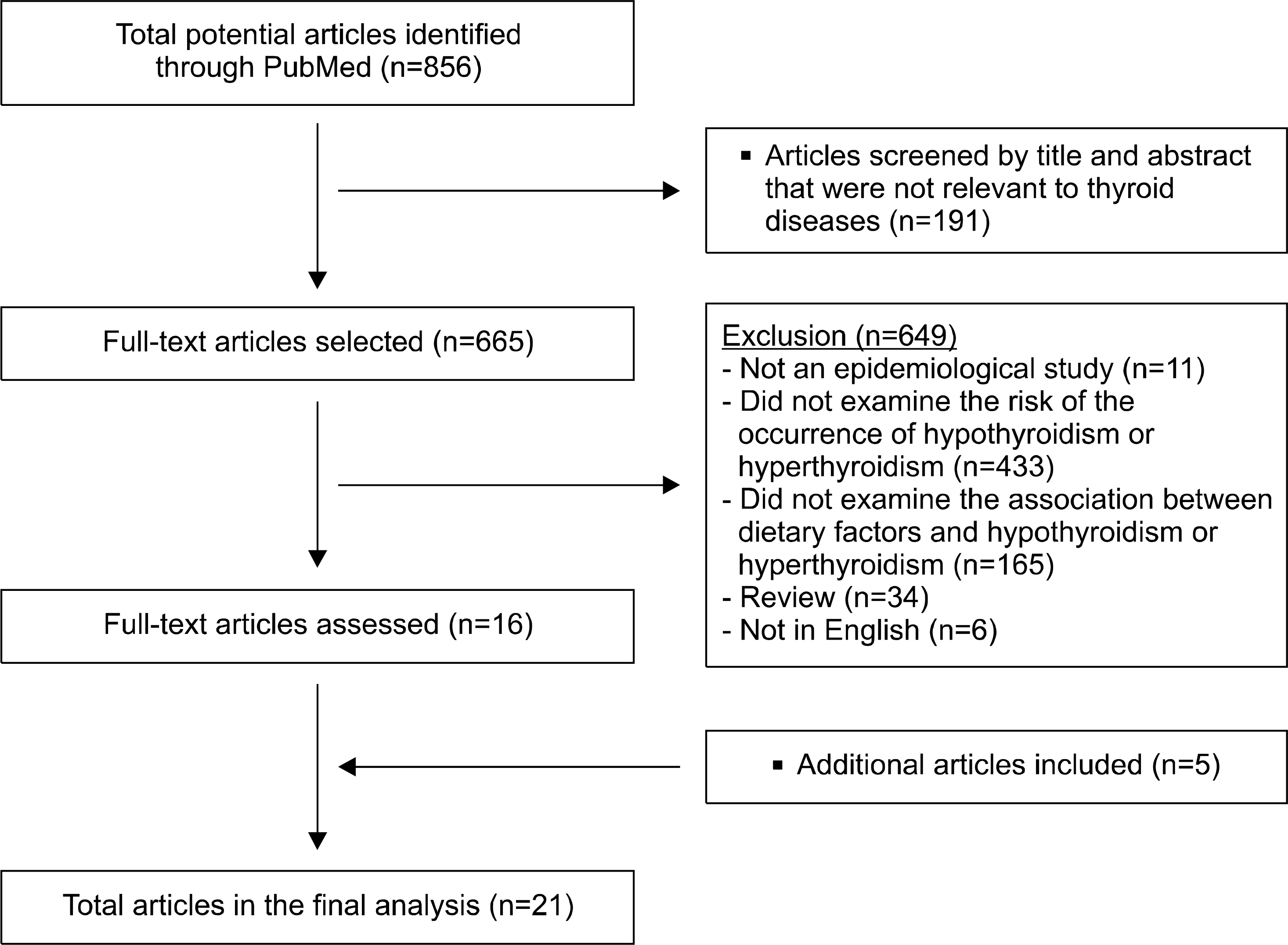
A total of 856 articles published between January 1st, 1990 and March 31st, 2015, were identified. Epidemiological studies that showed an association between dietary factors and thyroid diseases were selected, yielding a total of 21 articles.
Results
Due to a sudden increase in iodine supplementation (i.e., via salt iodization), regions such as Denmark and China, where insufficient iodine intake previously existed, showed a significant increase in the occurrence of hypothyroidism compared with that of hyperthyroidism. Other dietary factors, such as nitrate intake, may increase the risk of the diseases, whereas a vegan diet and alcohol intake may lower the risk.
References
1. World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed.Geneva: World Health Organization;2007.
2. Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. 2012; 142(4):744–50.

3. Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: Where do we stand in 2013? Thyroid. 2013; 23(5):523–8.

4. Michikawa T, Inoue M, Shimazu T, Sawada N, Iwasaki M, Sasazuki S, et al. Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Centerbased Prospective Study. Eur J Cancer Prev. 2012; 21(3):254–60.
5. Dahl L, Opsahl JA, Meltzer HM, Julshamn K. Iodine concentration in Norwegian milk and dairy products. Br J Nutr. 2003; 90(3):679–85.

6. Phillips DI. Iodine, milk, and the elimination of endemic goitre in Britain: the story of an accidental public health triumph. J Epidemiol Community Health. 1997; 51(4):391–3.

7. Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. 2010; 24(1):13–27.

8. Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-induced hypothyroidism. Thyroid. 2001; 11(5):): 501–10.

10. Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab. 1998; 83(3):765–9.

11. Carle A, Laurberg P, Pedersen IB, Knudsen N, Perrild H, Ovesen L, et al. Epidemiology of subtypes of hypothyroidism in Denmark. Eur J Endocrinol. 2006; 154(1):21–8.

12. Bulow Pedersen I, Knudsen N, Jorgensen T, Perrild H, Ovesen L, Laurberg P. Large differences in incidences of overt hyper- and hypothyroidism associated with a small difference in iodine intake: a prospective comparative register-based population survey. J Clin Endocrinol Metab. 2002; 87(10):4462–9.
13. Bulow Pedersen I, Laurberg P, Knudsen N, Jorgensen T, Perrild H, Ovesen L, et al. Increase in incidence of hyperthyroidism predominantly occurs in young people after iodine fortification of salt in Denmark. J Clin Endocrinol Metab. 2006; 91(10):3830–4.
14. Bulow Pedersen I, Laurberg P, Knudsen N, Jorgensen T, Perrild H, Ovesen L, et al. An increased incidence of overt hypothyroidism after iodine fortification of salt in Denmark: a prospective population study. J Clin Endocrinol Metab. 2007; 92(8):3122–7.
15. Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Carle A, Pedersen IB, et al. Lower prevalence of mild hyperthyroidism related to a higher iodine intake in the population: prospective study of a mandatory iodization programme. Clin Endocrinol (Oxf). 2009; 71(3):440–5.

16. Garcia-Garcia E, Vazquez-Lopez MA, Garcia-Fuentes E, Rodriguez-Sanchez FI, Munoz FJ, Bonillo-Perales A, et al. Iodine intake and prevalence of thyroid autoimmunity and autoimmune thyroiditis in children and adolescents aged between 1 and 16 years. Eur J Endocrinol. 2012; 167(3):387–92.
17. Yang F, Teng W, Shan Z, Guan H, Li Y, Jin Y, et al. Epidemiological survey on the relationship between different iodine intakes and the prevalence of hyperthyroidism. Eur J Endocrinol. 2002; 146(5):613–8.

18. Yang F, Shan Z, Teng X, Li Y, Guan H, Chong W, et al. Chronic iodine excess does not increase the incidence of hyperthyroidism: a prospective community-based epidemiological survey in China. Eur J Endocrinol. 2007; 156(4):403–8.

19. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006; 354(26):2783–93.

20. Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011; 164(6):943–50.

21. Zou S, Wu F, Guo C, Song J, Huang C, Zhu Z, et al. Iodine nutrition and the prevalence of thyroid disease after salt iodization: a cross-sectional survey in Shanghai, a coastal area in China. PLoS One. 2012; 7(7):): e40718.

22. Du Y, Gao Y, Meng F, Liu S, Fan Z, Wu J, et al. Iodine deficiency and excess coexist in china and induce thyroid dysfunction and disease: a cross-sectional study. PLoS One. 2014; 9(11):): e111937.

23. Konno N, Makita H, Yuri K, Iizuka N, Kawasaki K. Association between dietary iodine intake and prevalence of sub-clinical hypothyroidism in the coastal regions of Japan. J Clin Endocrinol Metab. 1994; 78(2):393–7.

24. Aminorroaya A, Janghorbani M, Amini M, Hovsepian S, Tabatabaei A, Fallah Z. The prevalence of thyroid dysfunction in an iodine-sufficient area in Iran. Arch Iran Med. 2009; 12(3):262–70.
25. Chung HR, Shin CH, Yang SW, Choi CW, Kim BI. Sub-clinical hypothyroidism in Korean preterm infants associated with high levels of iodine in breast milk. J Clin Endocrinol Metab. 2009; 94(11):4444–7.

26. Ward MH, Kilfoy BA, Weyer PJ, Anderson KE, Folsom AR, Cerhan JR. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. 2010; 21(3):389–95.

27. Tonstad S, Nathan E, Oda K, Fraser G. Vegan diets and hypothyroidism. Nutrients. 2013; 5(11):4642–52.

28. Carle A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, et al. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: a population-based case-control study. Eur J Endocrinol. 2012; 167(4):483–90.
29. Carle A, Bulow Pedersen I, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, et al. Graves' hyperthyroidism and moderate alcohol consumption: evidence for disease prevention. Clin Endocrinol (Oxf). 2013; 79(1):111–9.
30. Effraimidis G, Tijssen JG, Wiersinga WM. Alcohol consumption as a risk factor for autoimmune thyroid disease: a prospective study. Eur Thyroid J. 2012; 1(2):99–104.

31. Szabolcs I, Podoba J, Feldkamp J, Dohan O, Farkas I, Sajgo M, et al. Comparative screening for thyroid disorders in old age in areas of iodine deficiency, longterm iodine prophylaxis and abundant iodine intake. Clin Endocrinol (Oxf). 1997; 47(1):87–92.

32. Laurberg P, Jorgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, et al. The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol. 2006; 155(2):219–28.

33. Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid. 1998; 8(1):83–100.

34. Hetzel BS. Iodine deficiency disorders (IDD) and their eradication. Lancet. 1983; 2(8359):1126–9.

35. Moon S, Kim J. Iodine content of human milk and dietary iodine intake of Korean lactating mothers. Int J Food Sci Nutr. 1999; 50(3):165–71.
36. Parslow RC, McKinney PA, Law GR, Staines A, Williams R, Bodansky HJ. Incidence of childhood diabetes mellitus in Yorkshire, northern England, is associated with nitrate in drinking water: an ecological analysis. Diabetologia. 1997; 40(5):550–6.

37. Morales-Suarez-Varela MM, Llopis-Gonzalez A, Tejerizo-Perez ML. Impact of nitrates in drinking water on cancer mortality in Valencia, Spain. Eur J Epidemiol. 1995; 11(1):15–21.

38. Inoue-Choi M, Jones RR, Anderson KE, Cantor KP, Cerhan JR, Krasner S, et al. Nitrate and nitrite ingestion and risk of ovarian cancer among postmenopausal women in Iowa. Int J Cancer. 2015; 137(1):173–82.

39. Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004; 14(12):1012–9.

40. De Groef B, Decallonne BR, Van der Geyten S, Darras VM, Bouillon R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects. Eur J Endocrinol. 2006; 155(1):17–25.

41. Blount BC, Alwis KU, Jain RB, Solomon BL, Morrow JC, Jackson WA. Perchlorate, nitrate, and iodide intake through tap water. Environ Sci Technol. 2010; 44(24):9564–70.

42. McCarty MF. Vegan proteins may reduce risk of cancer, obesity, and cardiovascular disease by promoting increased glucagon activity. Med Hypotheses. 1999; 53(6):459–85.

43. Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2009; 32(5):791–6.

44. Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013; 23(4):292–9.

45. Leung AM, Lamar A, He X, Braverman LE, Pearce EN. Iodine status and thyroid function of Boston-area vegetarians and vegans. J Clin Endocrinol Metab. 2011; 96(8):E1303–7.

46. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013; 2013:965212.

47. Ott RA, McCall AR, McHenry C, Jarosz H, Armin A, Lawrence AM, et al. The incidence of thyroid carcinoma in Hashimoto's thyroiditis. Am Surg. 1987; 53(8):442–5.
Table 1.
Iodine intake and thyroid diseases in Europe
Table 2.
Iodine intake and thyroid diseases in Asia
Table 3.
Other dietary factors and thyroid diseases




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download