Abstract
Background
Bisphosphonate (BP) is an effective drug for the prevention and treatment of osteoporosis. However, gastrointestinal distress caused by BP is a well-known side effect for low compliance. The aim of our study was to compare the 1-year persistence, compliance and T-scores between the aperitif medication group and the postprandial medication group.
Methods
Three hundred patients were included in this study to determine their persistence and compliance with the prescribed daily BP (Maxmarvil®, alendronate 5 mg and calcitriol 0.5 µg; YuYu Pharm) following distal radius fractures. Patients in Group 1 (aperitif medication) were asked to adhere to the general guidelines for BPs before breakfast. Patients in Group 2 (postprandial medication) were recommended medication after breakfast. We compared the persistence and compliance of this daily BP therapy using the medication possession ratio (MPR) and T-scores between the 2 groups after 1 year.
Results
Bone mineral density in hip and lumbar spine was improved significantly in 2 groups (P<0.001). Significant differences existed between 2 groups, including 73 of 150 patients (48.7%) in Group 1, and 111 of 150 patients (73.3%) in Group 2 for 1-year persistence (P=0.001). The mean MPR is 0.66 in Group 1 (range, 0.50–0.86) and 0.71 in Group 2 (range, 0.54–0.87). A significant difference was detected between the 2 groups (P=0.002).
Osteoporosis is a systemic disease characterized by an increased risk of fracture due to low bone quality. Many drugs are available commercially in order to improve bone quality and prevent osteoporotic fractures. Among them, bisphosphonate (BP) is used as first-line medication for the treatment of osteoporosis. The outcome of BP treatment is affected by variable factors.[12] Medication adherence includes persistence (i.e., the length of time on therapy) and compliance (i.e., the consistency and accuracy with which the prescribed regimen is followed; medication possession ratio (MPR).[23]
Adherence to BP therapy may be lower than for the other drugs used to treat chronic diseases because patients with osteoporosis do not manifest any symptoms before fragility fractures occur.[4] Approximately 50% of all patients discontinue BP therapy within 1 to 2 years. Moreover, the percentage of patients showing high compliance (MPR >80%) at 1 and 2 years was only 44% and 39%, respectively.[456] The fracture prevention benefits of BPs cannot be realized by non-adherent patients. Conversely, the risk of fractures was reduced by 26% after 1 year of persistent BP use compared with non-persistence.[7] Therefore, the result of BP treatment depends on medication adherence.
Oral BP treatment involves a specific dosing regimen, which includes overnight fasting and upright position before and after administration, to improve bioavailability and decrease adverse effects.[8] However, these methods decrease adherence to BP consequently.[9] Therefore we established a hypothesis that adherence could be improved as changing dosing regimen. And we investigated whether adherence improved if patients did not comply with the dosing regimen of daily BP therapy. The aim of our study was to compare the compliance and 1 year persistence of daily BP, and T-score after 1 year between the aperitif medication and the postprandial medication.
This is a case-control study conducted with prospectively applied data.
Following approval by the Institutional Review Board (IRB; IRB No. SCHCA-2017-08-028), we included 300 patients who were prescribed daily oral alendronate, Maxmarvil® (alendronate 5 mg and Calcitriol 0.5 µg; YuYu Pharm, Inc., Seoul, Korea) from September 2012 to November 2017 for distal radius fractures (DRFs). In this study, patients were enrolled by starting treatment with an osteoporosis medication after a DRF, and they had not previously been treated for osteoporosis. The inclusion criteria were (1) age 60 years and above and treatment for DRFs; (2) diagnosis of osteoporosis (T-score below -2.5 standard deviation) based on the most recent bone mineral density (BMD) measurements (hip or spine, dual energy X-ray absorptiometry [DXA]); (3) without contraindications to oral BP; (4) without cognitive impairment; and (5) follow-up for 12 months or more after medication. Patients began taking a daily BP 1 week after trauma or surgery.
From September 2012 to September 2014, 150 patients with osteoporosis were recruited and investigated in the aperitif medication group (Group 1), and asked to adhere to the original guideline for BP therapy.
The propensity score matching with age and gender was used to design a control group of 150 patients, who had taken daily oral alendronate for the treatment of osteoporosis and were followed up for 12 months or more. The control group of patients was identified from patients treated with osteoporosis medication between October 2014 and November 2017. To obtain a postprandial control group, the dosing regimen was selected in accordance with the patients' preference. Patients who did not intend to comply with stringent dosing regimen (Group 2, postprandial medication) were recommended to take a medication after breakfast. Before selecting postprandial medication, we explained the adverse effects clearly and the possibility of poor T-score improvement compared with treatment with aperitif medication. Only patients who agreed to take postprandial medication were included in Group 2.
Parameters of age, gender, body mass index (BMI) were also collected. We calculated the Charlson comorbidity index (CCI) because the patients' dosing regimen was affected by medical comorbidity.
Patients who were willing to comply with stringent dosing regimen (Group 1, aperitif medication) were asked to adhere to the general BP guideline, requiring overnight fasting and maintenance of upright position. Patients who were unwilling to comply with stringent dosing regimen (Group 2, postprandial medication) were asked to take medication along with other medications for pain control, after their breakfast. All patients were followed up every 3 months including any loss to follow-up at specific time intervals. Following the definition of MPR, which is operationalized in retrospective assessment as the number of doses dispensed in relation to the dispensing period,[10] patients were asked to bring the remaining medications were not administered at each visit. Patients who failed to bring the remaining medications were asked about their number. The MPR was defined as the sum of administration days divided by 365 days after 1 year.
The patients' T-score was measured using DXA (GE lunar advance prodigy; Hologic Inc., Bedford, UK) before BP therapy. The T-scores of lumbar spine (mean of L1-4) and hip (femur neck or total femur) were measured. Patients who were persistence with their medication during 1 year were subjected to repeated DXA measurement. The number of patients who were lost follow-up at each visit was counted. Finally, the number of patients who maintained persistence in both groups was compared.
We compared the compliance and the persistence of this daily BP using MPRs and T-scores improvement in each group and between 2 groups after 1 year. And, we also reported gastrointestinal (GI) problems such as nausea, painful swallowing, dyspepsia, heartburn in 2 groups.
Statistical analysis was carried out using SPSS (version 21.0; IBM Corp., Armonk, NY, USA). An independent t-test was used to compare the continuous variables between the 2 groups. The significance of longitudinal changes in T-score for 1 year in each group was determined by paired t-test. A χ2 test was used to compare the persistence. Logistic regression was employed to assess the factors affecting the compliance and persistence. A significant level of P<0.05 was used for all comparisons.
The study enrolled a total of 300 patients: 150 belonging to Group 1 and another 150 belonging to Group 2. The differences in age, BMI and CCI between the 2 groups were also statistically significant. Demographic data of patients are summarized in Table 1.
Significant difference was not existed in 1-year lumbar and hip T-scores between 2 groups (P=0.131, 0.521, respectively). However there was a significant improvement in lumbar and hip T-scores in each group after 1 year (P<0.001, P<0.001, respectively). Lumbar T-score was significantly improved by 4.5% in Group 1, and 9.3% in Group 2 after 1 year. Hip T-score also improved by 4.2% in Group 1, and 3.8% in Group 2 (Table 2).
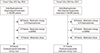
The mean cumulative persistence rate was 61.3% in all 300 patients. In Group 1, 73 of 150 patients (48.7%), and in Group 2, 111 of 150 (74%) patients were followed up and their T-score remeasured after 1 year. Significant differences in persistence were detected between both groups (P<0.001). Patients in Group 2 showed significantly higher persistence (Fig. 1, 2).
The mean MPR was 0.66 in Group 1 (range, 0.50–0.86) and 0.71 in Group 2 (range, 0.54–0.87). A significant difference was observed between the 2 groups (P=0.002). Factors that affect the 1 year persistence and MPR were not found by the logistic regression model when all variables were included stepwisely.
None of the patients had any fragility fracture during the 1 year after medication. No complications associated with daily bisphophonate medication were reported, including gastrointestinal effects in patients who were followed for 1 year. However, all patients who were dropped out refused to restart the treatment, because of abdominal pain or discomfort.
All medications have their own dosing regimen. Of medications, BP has very specific dosing regimen. This was developed for obtaining optimal bioavailability through pharmacokinetic experiments. Keeping stringent dosing regimen is one of the important factors to achieve therapeutic goal.
Besides, compliance and persistence are essential factors to ensure strong therapeutic adherence. Although many issues may affect adherence with BP, GI adverse effects are the most common reason for patient intolerance to oral BP. All patients who discontinued BP early in our study cited GI problems. Previous study also reported GI challenges were the most frequent adverse effects, which constitute discontinuation of therapy in about 46% of patients.[11] Furthermore, alendronate increased upper and lower GI bleeding risk after adjustment for age, sex, underlying comorbidity, and certain medications.[10]
Maxmarvil® is the enteric-coated alendronate, which reduces the GI problems comparatively. A comparative study used esophagogastroduodenoscopy for the evaluation of mucosal damage in the alendronate and Maxmarvil® groups without a history of GI issues. The Maxmarvil® group showed lower mucosal damage compared with the alendronate group.[12]
Considering the above, can we modify a dosing regimen slightly if comparable therapeutic result could be achieved and adverse effects could be reduced? This question was start of this study. As a result, Maxmarvil® improved bone mineral density, regardless of aperitif or postprandial intake in this study. Further study is needed to evaluated pharmacokinetics depends on dosing regimens.
Our study showed better result in the sphere of adherence using postprandial regimen. Previous study reported persistence of daily BP after 1, 3, and 5 years was 74.8%, 60.6%, and 51.7%, respectively. Low persistence was significantly associated with male sex, older age, and cyclical etidronate.[2] The cumulative persistence rate after one year was 61.3% (48.7% in Group 1, 73.3% in Group 2), which was lower to that of the previous study. Cumulative persistence rates are hard to directly compare across different studies, because of the higher mean age of patients in our study groups. Moreover, older age is a significant factor that reduces the persistence. Nevertheless, persistence of Group 2 was similar with previous study.
A meta-analysis reported a 46% increase in fracture risk over a 1- to 2.5-year follow-up when patients had MPR of <0.8.[13] A trend indicating an inverse relationship between compliance and fracture risk was found.[14] In our study, MPR in Group 1 and 2 was 0.66 and 0.71, respectively. Although only 24 patients maintained MPR over 0.8, no fragility fractures occurred.
We should address several limitations of our study. First, we did not perform a randomized study but used a historical control group, instead. A selection bias might exist in our study population, without interfering with the control group, which may be an ethical problem. Second, power analysis was not performed for calculating sample size. And, to identify statistical significance, the sample size was relatively small and follow-up duration was short. Although statistically similar groups were attempted to be matched, there was a statistically significant difference due to the relatively small number of patients. Third, we did not monitor or analyze the biomarkers of response to BP treatment. Therefore, a performance bias might exist because we did not examine vitamin D level, which affects the bone mineral density. As far, there is no study to evaluate adherence and bone mineral density depends on dosing regemen.
In this study, the postprandial daily BP reduced gastrointestinal discomfort, and represents an effective option to treat osteoporosis by improving compliance and persistence. If persistence and compliance to osteoporosis medication are less than 50%, osteoporosis treatment is not effective, and no fracture prevention benefits are found.[1516] Postprandial treatment with daily BP improved the persistence and the compliance, resulting in improved clinical outcomes.
Figures and Tables
Fig. 1
Flow chart for persistence of daily bisphosphonate in aperitif medication and postprandial medication. DRF, distal radius fractures; SERMs, selective estrogen receptor modulators.
Notes
AUTHOR CONTRIBUTION
Conceptualization: Park CH, Nho JH, Kim JH, Byun DW.
Data curation: Jung KJ, Kim JH, Chun DI.
Formal analysis: Park CH, Jung KJ, Won SH.
Methodology: Won SH, Chun DI, Kim JH, Byun DW.
Writing - original draft: Park CH, Kim JH, Won SH, Chun DI, Nho JH.
Writing - review & editing: Park CH, Jung KJ, Nho JH, Byun DW.
References
1. Curtis JR, Delzell E, Chen L, et al. The relationship between bisphosphonate adherence and fracture: is it the behavior or the medication? Results from the placebo arm of the fracture intervention trial. J Bone Miner Res. 2011; 26:683–688.


2. Ideguchi H, Ohno S, Hattori H, et al. Persistence with bisphosphonate therapy including treatment courses with multiple sequential bisphosphonates in the real world. Osteoporos Int. 2007; 18:1421–1427.

3. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008; 11:44–47.


4. Briesacher BA, Andrade SE, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008; 28:437–443.



5. Curtis JR, Westfall AO, Cheng H, et al. Benefit of adherence with bisphosphonates depends on age and fracture type: results from an analysis of 101,038 new bisphosphonate users. J Bone Miner Res. 2008; 23:1435–1441.



6. Nho JH, Lee YK, Ha YC, et al. Can alarming improve compliance with weekly bisphosphonate in patients with osteoporosis? J Bone Metab. 2016; 23:51–54.



7. van den Boogaard CH, Breekveldt-Postma NS, Borggreve SE, et al. Persistent bisphosphonate use and the risk of osteoporotic fractures in clinical practice: a database analysis study. Curr Med Res Opin. 2006; 22:1757–1764.


8. Gertz BJ, Holland SD, Kline WF, et al. Studies of the oral bioavailability of alendronate. Clin Pharmacol Ther. 1995; 58:288–298.


9. Hamilton B, McCoy K, Taggart H. Tolerability and compliance with risedronate in clinical practice. Osteoporos Int. 2003; 14:259–262.


10. Peng YL, Hu HY, Luo JC, et al. Alendronate, a bisphosphonate, increased upper and lower gastrointestinal bleeding: risk factor analysis from a nationwide population-based study. Osteoporos Int. 2014; 25:1617–1623.


11. Tosteson AN, Grove MR, Hammond CS, et al. Early discontinuation of treatment for osteoporosis. Am J Med. 2003; 115:209–216.


12. Mok JO, Jung CH, Kim CH, et al. Endoscopic comparison of alendronate alone and the enteric-coated alendronate with calcitriol combination in postmenopausal Korean females. Korean J Intern Med. 2013; 28:694–700.



13. Imaz I, Zegarra P, Gonzalez-Enriquez J, et al. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010; 21:1943–1951.


14. Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007; 18:271–277.






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download