Abstract
Background
Methods
Results
Conclusions
ACKNOWLEDGEMENT
References
Fig. 2
Cumulative incidence of fractures among the studied women with sphingosine 1-phosphate levels in the highest quartile compared with that in all other quartiles. CI, confidence interval.
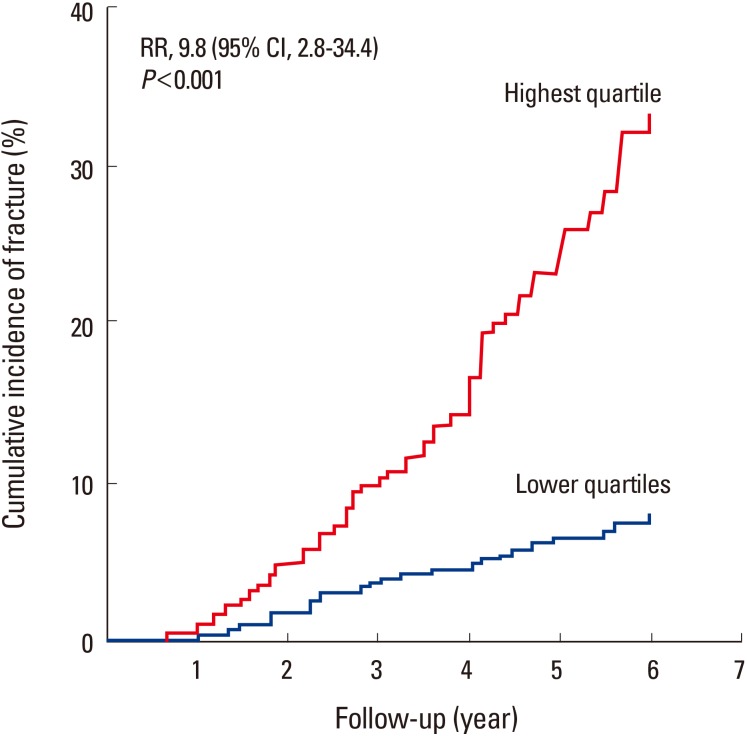
Fig. 3
Multivariable-adjusted hazard ratios for the risk of osteoporosis-related fractures, according to the quartile of plasma sphingosine 1-phosphate (S1P) concentration. Plasma S1P (µmol/L) quartiles were: Q1<4.85; Q2=4.86–5.24; Q3=5.25–5.81; and Q4≥5.82. The y axis is on a log scale. The reference group is quartile 1. The I bars denote 95% confidence intervals.
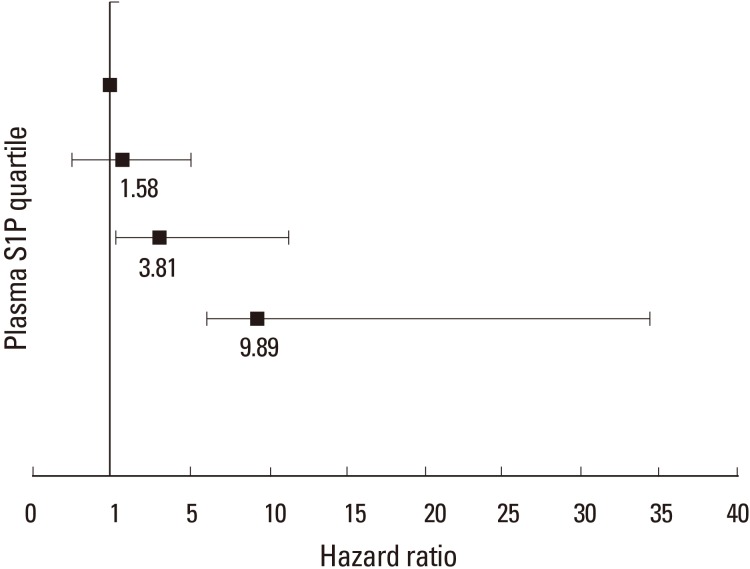
Table 1
Baseline characteristics of postmenopausal women studied according to fracture
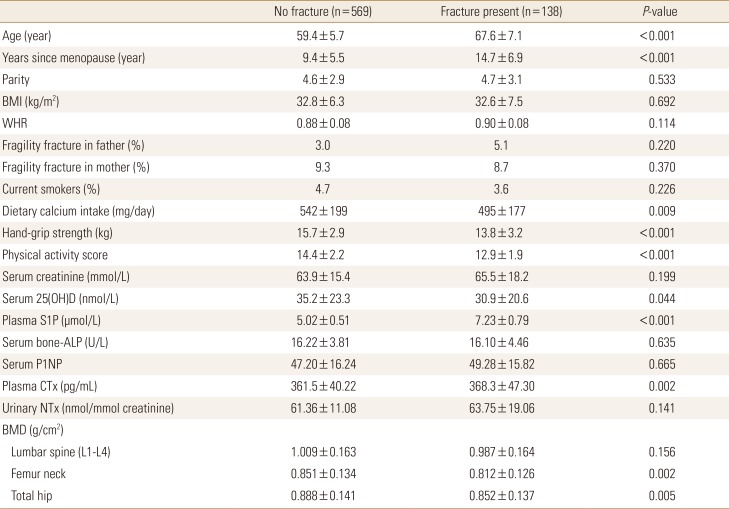
0.005The data is presented as mean±standard deviation.
To convert 25(OH)D values to ng/mL divide by 2.496; to convert creatinine values to mg/dL, divide by 88.4.
BMI, body mass index; WHR, waist-to-hip ratio; 25(OH)D, 25-hydroxy-vitamin D; S1P, sphingosine 1-phosphate; ALP, alkaline phosphatase; P1NP, procollagen type 1 N-terminal propeptide; CTX, C-terminal telopeptide of type 1 collagen; NTx, N-terminal telopeptide of collagen type I; BMD, bone mineral density.
Table 2
Plasma sphingosine 1-phosphate levels in the study participants at baseline and 1, 2, 3, 4, and 5 years
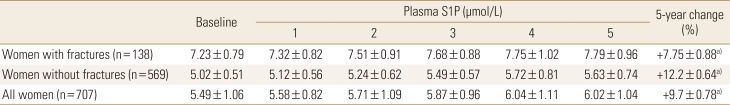
Table 3
Hazard ratios for osteoporosis-related fractures by sphingosine 1-phosphate cut-off values
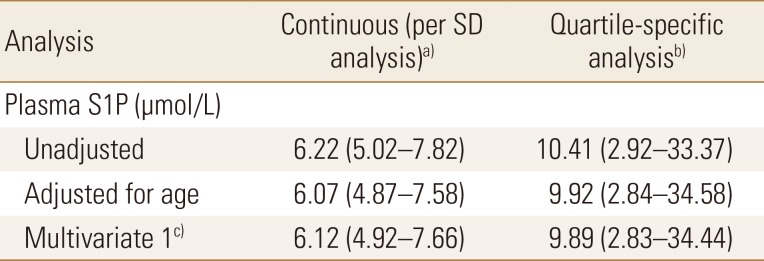
The data is presented as HR (95% confidence interval) and all values were statistically significant.
a)S1P levels were analyzed as a continuous measure. The HR is for each increment of 1-SD above the mean value. b)S1P levels were analyzed as a dichotomous variable. The HR for women in the highest quartile of S1P levels, as compared with women in the three lower quartiles. c)The analysis included adjustments for age, body mass index, physical activity score, dietary calcium intake, serum 25(OH)D, hand-grip strength, and bone mineral density of total hip.
SD, standard deviation; S1P, sphingosine 1-phosphate; HR, hazard ratio; 25(OH)D, 25-hydroxy-vitamin D.
Table 4
Correlation between plasma sphingosine 1-phosphate levels and bone mineral density at various sites, bone turnover markers and other potential confounding factors among postmenopausal women studied
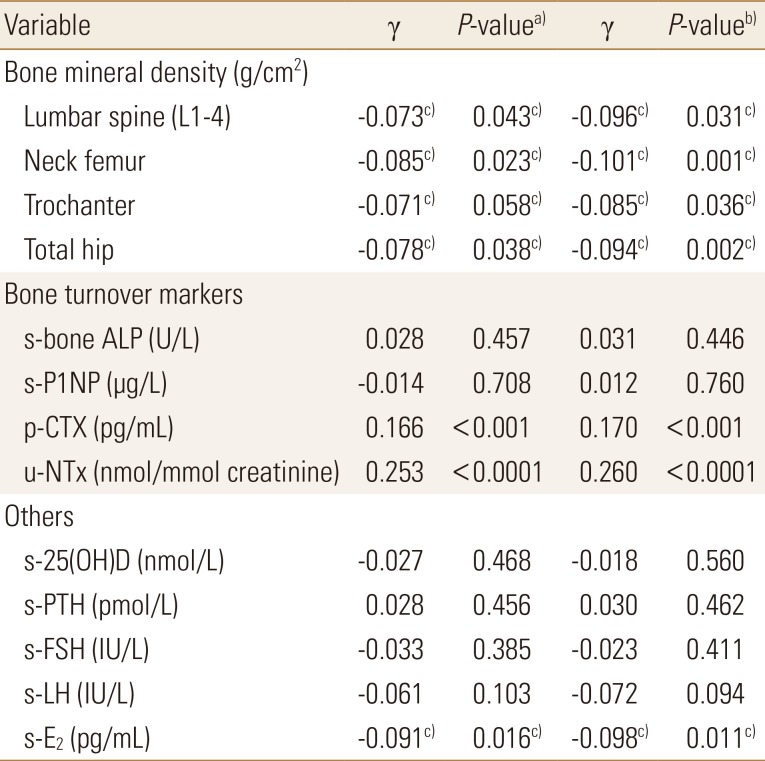
To convert 25-hydroxyvitamin-D values to ng/mL divide by 2.496.
a)P-values were determined by Pearson's correlation analysis with respect to plasma S1P levels. b)P-values were determined by partial correlation analysis with respect to plasma S1P levels adjusted for age, body mass index, physical activity-score, current smoking, hand-grip strength and dietary calcium intake. c)The statistically significant.
s-bone ALP, serum bone alkaline phosphatase; s-P1NP, serum procollagen type 1 N-terminal propeptide; p-CTX, plasma C-terminal telopeptide of type 1 collagen; u-NTx, urinary N-terminal telopeptide of collagen type I; s-25(OH)D, serum 25-hydroxy-vitamin D; s-PTH, serum parathyroid hormone; s-FSH, serum follicle stimulating hormone; s-LH, serum luteinizing hormone; s-E2, serum estradiol; S1P, sphingosine 1-phosphate.
Table 5
Hazard ratio with 95% confidence interval per standard deviation or highest quartile of plasma sphingosine1-phosphate levels (µmol/L) for osteoporosis-related fractures from baseline (time point zero) to 2, 3, and 5 years after baseline
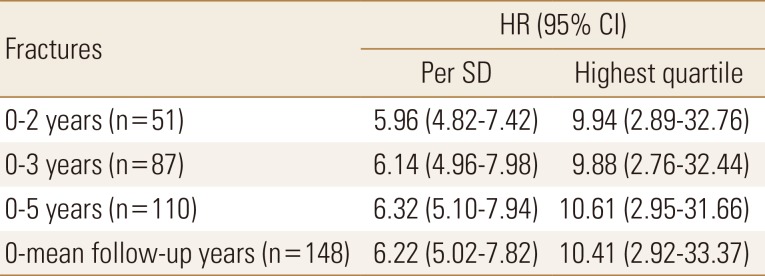
All values were statistically significant. Women with fractures are compared with women without fractures. Follow-up starts from baseline visit, and the annual follow-up times are 2, 3, and 5 years and the complete period was 5.2±1.3 years. HR is given per SD increase or per highest quartile of plasma sphingosine 1-phosphate (µmol/L). The lowest quartiles are used as the reference group (HR, 1.0).
HR, hazard ratio; CI, confidence interval; SD, standard deviation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download