Abstract
Steroid-induced osteoporosis is the most common cause of secondary osteoporosis and accounts for one-fifth of all osteoporosis cases. The fracture incidence under steroid may be as high as 50%. However, many patients do not undergo appropriate risk assessment and treatment before and after steroid exposure. We described a 56-year-old male patient with multiple punched-out lesions in skull unusually as well as vertebral, fibular, rib and humeral fractures during steroid use without proper management.
Steroids play an important role in the treatment of various diseases, but adverse effects are common. Particularly, osteoporosis is severe adverse effect of steroid treatment. Steroid-induced osteoporosis is the leading cause of secondary osteoporosis. Approximately one out of five patients with osteoporosis are known to possess steroid-induced osteoporosis.[1] Bone loss caused by steroid occurs in both trabecular and cortical bone, however it remarkably affect in the parts of the skeleton that have trabecular bone such as vertebrae and ribs.[2] Fractures occur in 30-50% of patients who had long-term steroid treatment and vertebral fracture is the most common.[3] So far, the cases regarding fractures caused by steroid therapy are mostly multiple vertebral fractures or hip fracture. To the best of our knowledge, there have been few cases with multiple punched-out lesions caused by steroid treatment, typical radiologic findings of multiple myeloma, in the skull containing abundant cortical bones.
Here, in order to emphasize the importance of appropriate prevention and treatment of steroid-induced osteoporosis, we report the very rare case of middle-aged male with multiple fractures in several parts of his body and punched-out lesions in the skull due to long-term steroid use, without other causes of secondary osteoporosis.
A 56-year-old Korean male was referred to our hematology department for the evaluation of multiple myeloma. He had an extensive history of repeated and multiple vertebral fractures. Three years ago, the patient experienced pain in his lower back and on his bilateral hip pain without any particular injury history and was treated with analgesics. Six months ago, the symptoms progressed and became intolerable in spite of analgesia. Magnetic resonance imaging (MRI) confirmed compression fractures at 9th and 12th thoracic vertebrae and a percutaneous vertebroplasty was performed to help relieve the pain. A month ago, radiographs showed new compression fractures at 8th and 9th thoracic vertebrae and the percutaneous vertebroplasty was performed again (Fig. 1A, 1B). Unfortunately, ten days ago, back pain redeveloped and lower extremity weakness occurred. MRI showed spinal cord compression as well as mass effect at 8th and 9th thoracic vertebrae. Also, compression fractures at 10th thoracic vertebra were newly observed. According to the patient's medical history, he was diagnosed with hypertension 3 years ago and he has been taking medications occasionally. He tended to drink alcohol of 160 g per week for about 20 years and had a habit of smoking about 20 pack-year. Although, the patient had stable vital signs, his physical examination had shown that he had chronic ill-looking appearance and a Cushingoid appearance with moon face, easy bruising, thin skin and atrophy of upper leg muscles. Manual muscle testing of lower extremity showed weakness of grade 3-3+ out of 5.
Our laboratory work-up including complete blood cell count, liver and renal function test, serum electrolytes and blood glucose did not show any abnormal results except the neutrophilia and elevation of erythrocyte sedimentation rate (ESR) (hemoglobin: 14.5 g/dL, thrombocyte: 192,000/mm3, white blood cell: 6,9701/mm3 [neutrophil; 80%], ESR: 32 mm/h [reference value: 0-15], blood urea nitrogen: 11 mg/dL, creatinine: 0.63 mg/dL, total protein: 6.5 g/dL, albumin: 3.8 g/dL, calcium: 8.9 mg/dL [reference value: 8.0-10.0], phosphate: 2.9 mg/dL [reference value: 2.6-4.5], intactparathyroid hormone: 27.23 pg/mL [reference value: 13.0-54.0]). Simple radiography revealed calcified granuloma on the right upper lung, multiple punched-out lesions in the skull, healed fracture of the left febula, multiple rib fractures, kyphosis of the thoracic vertebrae and lesions of bone cement injections at 8th, 9th, and 12th thoracic vertebrae (Fig. 2A, 2B, 2C). Computed tomography (CT) and MRI of the spine showed multiple compression fractures and spondylitis with left paraspinal and anterior subligamentous abscesses (Fig. 3A). In the evaluation of the multiple myeloma, serum and urine protein electrophoresis were normal and bone marrow biopsy revealed only granuloma consistent with mycobacterial infection. Tumor markers (alpha-fetoprotein [AFP], prostate-specific antigen [PSA], cancer antigen [CA]125, CA19-9, carcinoembryonic antigen [CEA]) were normal. On the CT of chest and abdomen, there were small nodules and branching linear structure, suggesting pulmonary tuberculosis, on the both sides of upper lung, calcified granuloma on the right apex, and multiple osteolytic lesions with vertebral collapse. In bone biopsy, necrotic bone fragment and hemorrhage was seen and tuberculosis (TB)-polymerase chain reaction (PCR) was positive.
The patient was referred to the endocrinology because there was no evidence of multiple vertebral compression fractures cause by multiple myeloma and malignancy and he had a Cushingoid appearance. We found that he had past medical history included psoriasis managed by intramuscular self-injection of triamcinolone since 5 years ago; 6 cc of triamcinolone injected 5 times in the first year and then 3 cc of triamcinolone was injected twice per month for the following 4 years. Values of 30 and 60 minutes post-250 µg-injection adrenocorticotropic hormone were 0.94 µg/dL and 4.42 µg/dL, respectively, indicating adrenal insufficiency. A dual-energy X-ray absorptiometry (DXA) revealed osteoporosis (L2-L4 T-scores = -4.7, femur neck T-score = -3.8) (Fig. 3B). In bone turnover maker, bone-specific alkaline phosphatase (ALP) was raised (36.00 µg/L, reference value: 6.00-30.00) and osteocalcin was decreased (8.79 ng/mL reference value: 13.0-28.0). 25(OH) vitamin D3 measured by enzyme-linked immunosorbent assay (ELISA) was 5.4 ng/mL, indicating vitamin D deficiency.
The patient was received non-steroid anti-inflammatory analgesic drugs (NSAIDs) in order to relieve pain and 7.5 mg of prednisolone orally to manage adrenal insufficiency. For the treatment of osteoporosis, vitamin D3 (1,000 IU daily), elementary calcium (500 mg daily) and risedronate (35 mg weakly) were given. Also, he was treated with antituberculosis medication. Unfortunately, six days after his discharge from the hospital, and on his way back home from the first out-patient visit, he had fallen down on his right side and had the proximal humerus fracture. Then, he received conservative treatment (Fig. 2D). Two months after the medical treatment, osteocalcin decreased (5.27 ng/mL) and serum C-terminal telopeptide (CTx) was lower than the normal value (0.375, reference value < 0.7 ng/mL) and 25(OH) vitamin D3 increased 14.3 ng/mL.
In many cases, secondary causes of bone loss are often missed in the patients who were diagnosed osteoporosis. According to many studies, the secondary causes are present in 20-30% of postmenopausal women and more than 50% of premenopausal women and men who were diagnosed osteoporosis.[4] Moreover, a retrospective study performed on the patients with fragility fractures reported that 54% of the patients showed either vitamin D insufficiency or deficiency and about one-third of the rest had the secondary causes of osteoporosis other than vitamin D inadequacy.[5]
Steroids are the most common cause of secondary osteoporosis and steroid-induced osteoporosis represents 20% of whole patients with osteoporosis.[1] Often, like the present case, the first presenting manifestation is a fracture, which occurs in 30-50% of patients receiving long-term steroid treatment; vertebral fracture is the most common.[3] According to the large scale meta-analysis investigating the patients with steroid treatment, it has been reported that the relative risk of fractures are increased by 2.86, 1.61, 1.13, and 1.91 for vertebrae fractures, hip fractures, wrist fractures, and total fractures, respectively.[6]
Bone loss occurs soon after steroid therapy and progresses rapidly within first 3 months and peaks at 6 months, followed by a slower, steady state with continued use.[7] Although both types of bone are affected, the more rapid loss occurs in trabecular bone. Steroids have both direct and indirect effects on the skeletal system.[1,8,9] Steroids stimulate osteoclast-mediated bone resorption and reduce osteoblast-mediated bone formation. They inhibit osteoblast lineage differentiation into osteoblast, enhance osteoblast lineage differentiation into adipocytes, decrease osteoblast function and increase osteoblast apoptosis, resulting in impaired bone formation. In addition, steroids increase formation of osteoclasts and decrease apoptosis of mature osteoclasts, inducing a prolonged and enhanced bone resorption.[1,8,9] Indirect effects include a decrease of intestinal calcium absorption and an increase of renal calcium excretion. This can results in secondary hyperparathyroidism, which in turn leads to increased bone turnover. Furthermore, steroids decrease the secretion of sex hormone by reducing the release of gonadotropins, inducing bone resorption.[1,8,9] There are many risk factors associated steroid-induced osteoporosis and related fracture; old age, low bone mineral density (BMD), low body mass index, underlying disease affecting bone metabolism, individual sensitivities against steroids, the family history of hip fractures, excessive drinking and smoking, frequent fall and the history of fractures.[2]
In the present case, patient was referred to the hematologist for evaluation of the repeated and multiple vertebral fractures, under the impression of multiple myeloma. Although he had no trauma and fracture history, multiple osteolytic lesions in the skull and generalized fractures found. There was no evidence of multiple myeloma and malignancy but steroid use, which can affect his bone lesions. According to reports, osteoporotic fractures after steroid treatment are mostly vertebral fractures or hip joint fractures because steroid-induced bone loss occurs predominantly in trabecular bone. However, it can occur in cortical bone. There are two cases of bilateral tibia and fibula fractures and metatarsal bone fractures associated with steroid therapy.[10,11] After steroid use, the present case had multiple punch-out lesions, demonstrating multiple myeloma, in the skull with dense cortical bone that resists resorption. Also, he experienced newly fragility fracture of the proximal humerus during follow-up.
Many patients receiving steroid therapy do not receive appropriate interventions to prevent or treat osteoporosis, although steroids have been widely used for many clinical purposes. Majumar et al.[12] reported the prevention and management of osteoporosis for the patients who newly received the long-term steroid treatment for 10 years (1998 thru 2008). In results, although the prevention of steroid-induced osteoporosis was improved over time, it was found that only 25% of patients received a BMD test or osteoporosis treatment within 6 months of steroid administration.[12] There are several guidelines for the prevention and treatment of steroid-induced osteoporosis. According to the recommendations of American College of Rheumatology (ACR) for the prevention and treatment of steroidinduced osteoporosis in 2010, including the 2001 recommendation, it was suggested to consider fall risk assessment, height and 25-hydroxyvitamin D measurement, evaluation for prevalent and incident fragility fractures, vertebral fracture assessment or radiographic imaging of the spine and calcium and vitamin D supplementation for any duration of steroid use.[7]
In Korea, there is lack of data on steroid use and steroid-induced osteoporosis and related fractures. Furthermore, general practitioners as well as patients have received less attention. Therefore, here, we report this case with multiple punch-out lesions in the skull after long-term steroid use in order to address the significance of prevention and treatment of steroid-induced osteoporosis.
Figures and Tables
Fig. 1
(A) Six months ago, sagittal T2-weighted MRI of the thoracic spine showed acute compression fractures at T9 and T12. (B) After kyphoplasty, sagittal T2-weighted MRI of the lumbar spine showed acute compression fractures at T8, T10 and T11 and prominent fracture lines at T9 and T12.

Fig. 2
(A) Lateral radiograph of the skull demonstrates multiple punched-out lesions with variable sizes on the parietal region. (B) Lateral radiograph of thoracic spine shows kyphosis of the thoracic vertebrae and multiple compression fractures with vertebroplasty segments at T8 through T12. (C) Posteroanterior plain radiograph demonstrates a left healed fibular fracture.

Fig. 3
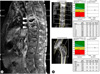
(A) Sagittal fat-suppressed contrast-enhanced T2 weighted MRI of the spine shows spondylitis of T8, 9, 10 with left paraspinal and anterior subligamentous abscesses (arrow head) and compression fractures at T8 through L5. (B) Low bone mineral density at lumbar spine and left femur is demonstrated, indicating "osteoporosis". AP, anteroposterior; BMD, bone mineral density.
References
2. Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011. 365:62–70.
3. McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008. 20:131–137.

5. Bogoch ER, Elliot-Gibson V, Wang RY, et al. Secondary causes of osteoporosis in fracture patients. J Orthop Trauma. 2012. 26:e145–e152.

6. van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002. 13:777–787.

7. Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2010. 62:1515–1526.

9. Canalis E. Mechanisms of glucocorticoid-induced osteoporosis. Curr Opin Rheumatol. 2003. 15:454–457.

10. Avancini-Dobrović V, Vrbanić TS, Kukuljan M, et al. Spontaneous serial fractures of metatarsal bones in female patient with rheumatoid arthritis on long-term steroid therapy. Coll Antropol. 2010. 34:1123–1126.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download