Abstract
Serum consists of water (93% of serum volume) and nonaqueous components, mainly lipids and proteins (7% of serum volume). Sodium is restricted to serum water. In states of hyperproteinemia or hyperlipidemia, there is an increased mass of the nonaqueous components of serum and a concomitant decrease in the proportion of serum composed of water. Thus, pseudohyponatremia results because the flame photometry method measures sodium concentration in whole plasma. A sodium-selective electrode gives the true, physiologically pertinent sodium concentration because it measures sodium activity in serum water. Whereas the serum sample is diluted in indirect potentiometry, the sample is not diluted in direct potentiometry. Because only direct reading gives an accurate concentration, we suspect that indirect potentiometry which many hospital laboratories are now using may mislead us to confusion in interpreting the serum sodium data. However, it seems that indirect potentiometry very rarely gives us discernibly low serum sodium levels in cases with hyperproteinemia and hyperlipidemia. As long as small margins of errors are kept in mind of clinicians when serum sodium is measured from the patients with hyperproteinemia or hyperlipidemia, the present methods for measuring sodium concentration in serum by indirect sodium-selective electrode potentiometry could be maintained in the clinical practice.
In the diagnostic approach for the patients with hyponatemia, pseudohyponatremia should always be differentiated during the initial steps1). Although many clinicians are familiar with the term "pseduhyponatremia", they may be aware of the concept and application of pseudohyponatremia only on rare occasions during their practice.
Pseudohyponatremia is an exaggeration of the physiologic dilution of plasma sodium by nonaqueous material2). High concentrations of intravascular protein (as in multiple myeloma) or lipid "dilute" the plasma sodium concentration, but do not alter the solute concentrations of the intracellular or interstitial fluid compartments. Because the "diluent" in these conditions is nonaqueous, the sodium concentration of the aqueous portion of a plasma sample, as determined by an ion-selective electrode in undiluted serum or plasma, is normal3). Plasma osmolality, as measured by an osmometer, also is normal. Thus hyponatremia associated with hyperlipidemia or hyperproteinemia can be considered artifactual, and is called pseudohyponatremia4).
Previously, Fuisz refered to this pseudohyponatremia as a well-known matter in a very comprehensive study on hyponatremia5). It should be noted that laboratory determinations that depend on a diluting step yield artifactually low sodium concentrations in samples taken from patients with severe hyperlipidemia or plasma cell dyscrasias, even if the instrument uses an ion-selective electrode6). In these disorders, the plasma aliquot obtained for dilution contains less plasma water (and therefore less sodium) than normal; the sodium concentration in the diluted sample is thus artifactually reduced. These notions can hardly be realized in daily clinical practice, and pseudohyponatremia may need to be reappraised from the current clinician's viewpoint.
A 35 year-old black male soldier was admitted due to lower extremity weakness. He was previously healthy until he had upper respiratory infectious symptoms 3 days prior to admission. After admission to the department of neurology his motor weakness progressed towards upper extremities. Initial serum electrolyte profile showed Na 131, K 3.4, Cl 96, and total CO2 25.9 mEq/L. Clinical diagnosis of Guillain-Barre syndrome was made, and intravenous immunoglobulin therapy was carried out for 5 days. At the end of the intravenous infusion of immunoglobulin, his serum sodium concentration was decreased to 116 mEq/L but without any relevant symptoms. His serum total protein was 10.3 g/dL at that time.
Hyponatremia is a known complication associated with the administration of intravenous immunoglobulin (IVIG)7-11). Because IVIG therapy results in post-infusional hyperproteinemia or an increase in the nonaqueous phase of plasma, IVIG is thought to cause pseudohyponatremia, rather than true hyponatremia. Steinberger et al. conducted a prospective observational study to evaluate the incidence of hyperproteinemia occurring after IVIG therapy and its relationship to serum sodium, osmolality, and the serum osmolal gap10). Eighteen IVIG infusions at a standard dose of 2 g/kg administered over 2-5 days were evaluated. Paired t-testing revealed a statistically significant increase in serum protein and viscosity and decrease in serum sodium and calculated osmolality 24 hr after the completion of IVIG therapy (Fig. 1). The calculated serum osmolal gap increased insignificantly.
We suspected that our patient had pseudohyponatremia due to IVIG-associated hyperproteinemia. However, measurement of serum osmolality and determination of serum sodium concentrations with both indirect and direct ion-selective electrodes (ISE) were necessary to exclude the possibility of true hyponatremia. The emergency laboratory of my hospital uses two models of autoanalyzers for measurement of electrolytes. The one is Hitachi 7170S, and the other Dade Behring Dimension. Both use ion-selective electrodes in diluted plasma specimens (indirect ISE, 30×dilution for Hitachi 7170S and 10×dilution for Dade Behring Dimension) for measurement of sodium concentration.
IVIG therapy can result in true hyponatremia, resulting from sucrose-induced translocation of water from the intracellular compartment to the extracellular compartment, as well as the infusion of a large volume of dilute fluid12). However, this occurs in patients with an underlying defect in urinary free-water excretion such as advanced chronic kidney disease. Our patient had no azotemia and seemed to have no defect in free-water excretion.
Besides, an association of the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) with GuillainBarre syndrome has been clearly documented in a number of previous reports13-16). According to Hoffmann et al., 25% of patients with Guillain-Barre syndrome present with hyponatremia due to SIADH17). The result of low serum osmolality would be a differential clue to SIADH.
Traditionally, sodium in plasma or serum has been determined by flame photometry. Nowadays, most diagnostic laboratories in the hospital use sodium-selective electrodes. The development of electrodes that are selective for sodium opened the possibility of potentiometric determination18), which offers advantages. Plasma is normally 93% water, and the sodium ion concentration in "plasma water" is the biological index. Thus a sodium concentration of 143 mEq/L in a sample of whole plasma reflects a sodium concentration of 154 mEq/L in plasma water (143/0.93=154). Physiologic saline solutions (for example, 0.9% NaCl) were designed to reproduce the sodium concentration in plasma water2).
Ion-selective electrodes for the analysis of sodium are used via two major approaches (Fig. 2)19). In one, the sample is diluted before analysis (indirect potentiometry), analogous to flame photometry. In the other, the sample is analyzed without dilution (direct potentiometry), analogous to blood pH measurement. When using indirect potentiometry, if the diluent and dilution ratio are properly selected, the activity coefficient and residual liquid junction potential between standards and samples should be matched and the results should reflect total concentration. Therefore, it is not surprising that indirect potentiometry and flame photometry give the same values for sodium in plasma with various instruments.
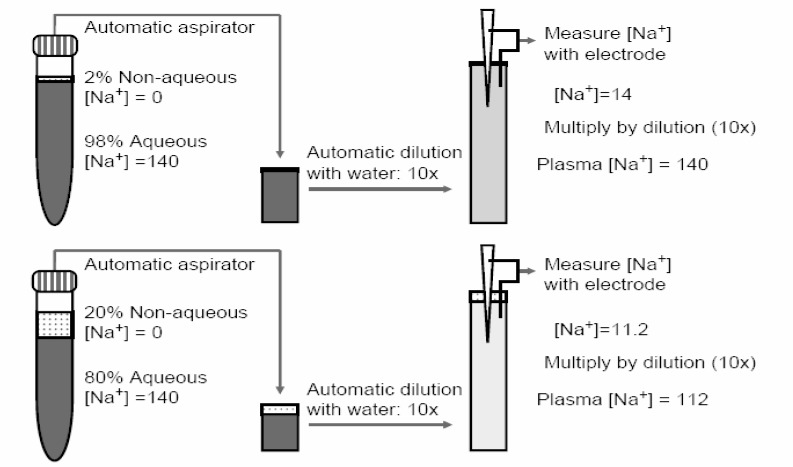
Direct-potentiometric sodium values are not expected to be the same as flame photometric values because of the influence of water content as depicted in Fig. 319). In clinical conditions such as hyperlipemia or hyperproteinemia, in which the water content of pl asma is decreased, low flame-photometric sodium values have been long noted. Under such conditions, direct-potentiometric values are more clinically appropriate. Given that the water content of normal serum is 91-93%, a technique that utilizes a fixed volume aliquot (flame photometry or indirect potentiometry) should give values 7-9% lower than a technique that does not involve fixed-volume aliquots (direct potentiometry) when both use standardization with NaCl. This is because direct potentiometry should measure the activity of sodium only in the water phase. However, sodium values obtained by direct potentiometry and flame photometry show that flame-photometric values are 95.7 to 98.8% of direct-potentiometric values20). Indirect potentiometry also can lead to an underestimation of serum sodium even though the sodium activity registered by the sodium-selective electrode is oblivious to the non-aqueous volume. This is explained in Fig. 4. Automatic aspirators and dilutors prepare the samples and, in situations with excessive non-aqueous volume in plasma, a smaller than expected plasma volume may be aspirated leading to an underestimation of serum sodium during the back-calculation21).
Extreme cases of hyperlipemia22) and hyperproteinemia23) will simulate hyponatremia, i.e., spuriously low sodium concentrations will be determined in sera that are markedly lactescent or for sera with the extremely high protein values that are typical in multiple myeloma. In these instances the direct method will give values for sodium that are nearer the normal. Then will the indirect method mislead us to confusion in interpreting the serum sodium data?
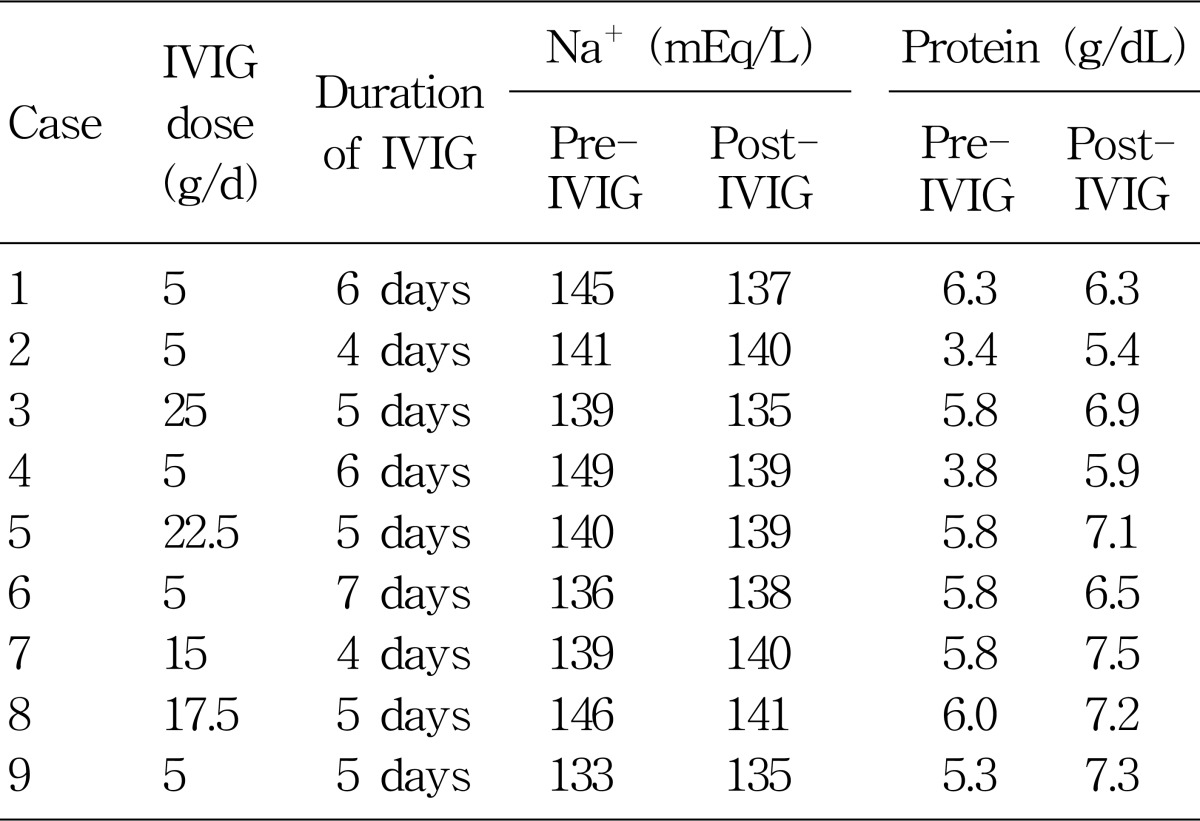
My hospital uses indirect ISE methods (Hitachi and Dade Behring) for the measurement of serum sodium concentration. Serum chemistry data were retrospectively investigated from 9 patients who were admitted to my hospital and received IVIG infusion for 4 to 7 days during the recent 6 months (Table 1). The indications for IVIG were variable: neurologic, infectious, autoimmune, and inflammatory disorders. Doses of IVIG were variable from 5 to 25 g/day. After the IVIG infusion, serum total protein was significantly increased from 5.33±1.02 (mean±SD) to 6.68±0.71 g/dL. However, serum sodium concentration did not show any significant change between pre- and post-IVIG infusion : 141±5 mEq/L vs. 138±2 mEq/L.
Steinberger et al. nicely demonstrated that pseudohyponatremia occurred after IVIG infusion therapy10). They administered a standard dose of 2 g/kg over 2 or 5 days. After the IVIG infusion, serum total protein was significantly increased from 6.32 to 8.15 g/dL. Serum sodium concentration showed a "significant" decrease from 136 to 133 mEq/L. It is interesting that the mean decrease in serum [Na+] is almost the same (-3 mEq/L) as ours.
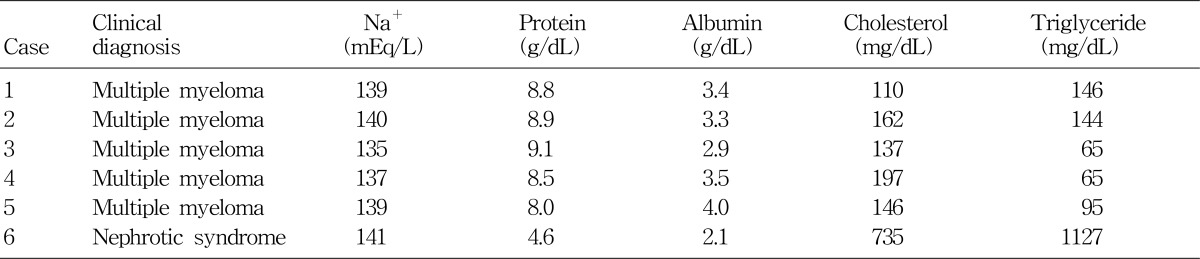
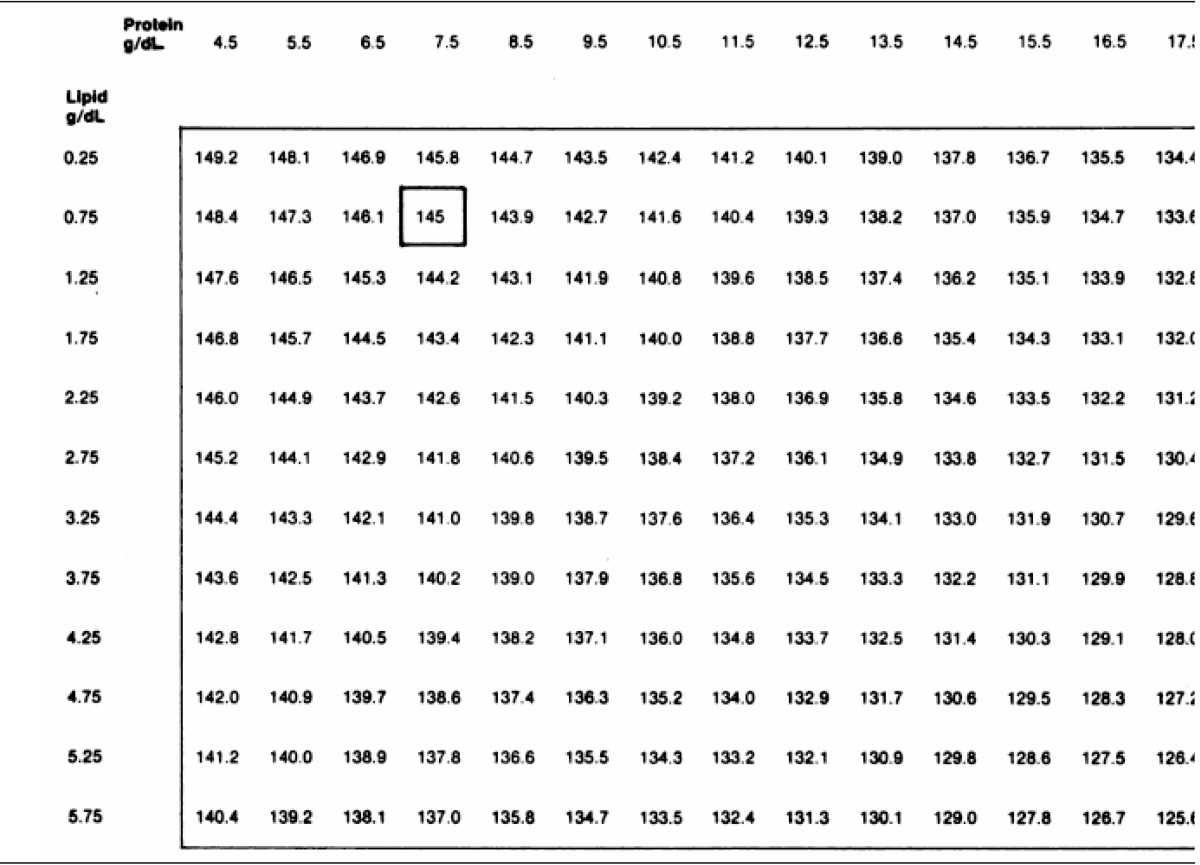
Table 2 shows serum chemistry data from 5 patients with multiple myeloma and 1 with nephrotic syndrome, and they were also admitted to my hospital during the recent 6 months. All 4 myeloma patients had rather high levels of serum total protein, but their serum sodium concentrations were not notably low. The patient with nephrotic syndrome (Case 6) presented with severe hyperlipidemia, but his serum sodium concentration was within normal range. Although hyperlipidemia causes errors in indirect ISE electrolyte measurements, the decrease in serum [Na+] would be only -1 mEq/L for each 10-mmol/L increase in total lipid concentration24). Actually, as seen in the Table 3 which was proposed by Levy25) for the correction of errors caused by hyperproteinemia or hyperlipidemia, the margins of errors do not seem to be quite discernible.
References
1. Chonchol M, Berl T. DuBose TD, Hamm LL, editors. Hyponatremia. Acid-base and electrolyte disorders. 2002. Philadelphia: WB Saunders;p. 229–239.
2. Sterns RH, Silver SM, Spital A. Seldin DW, Giebisch G, editors. Hyponatremia. The kidney. Physiology & pathophysiology. 2000. Philadelphia: Lippincott Williams & Wilkins;p. 1217–1238.

4. Albrink MJ, Hald PM, Man EB, Peters JP. The displacement of serum water by the lipids of hyperlipemic serum; a new method for the rapid determination of serum water. J Clin Invest. 1955; 34:1483–1488. PMID: 13263427.
6. Ladenson JH, Apple FS, Koch DD. Misleading hyponatremia due to hyperlipemia: a method-dependent error. Ann Intern Med. 1981; 95:707–708. PMID: 7305152.

7. Koffman BM, Dalakas MC. Effect of high-dose intravenous immunoglobulin on serum chemistry, hematology, and lymphocyte subpopulations: assessments based on controlled treatment trials in patients with neurological diseases. Muscle Nerve. 1997; 20:1102–1107. PMID: 9270664.

8. Lawn N, Wijdicks EFM, Burritt MF. Intravenous immune globulin and pseudohyponatremia. N Engl J Med. 1998; 339:632. PMID: 9722438.

9. Ng SK. Intravenous immunoglobulin causing pseudohyponatremia. Lupus. 1999; 8:488–490. PMID: 10483022.
10. Steinberger BA, Ford SM, Coleman TA. Intravenous immunoglobulin therapy results in post-infusional hyperproteinemia, increased serum viscosity, and pseudohyponatremia. Am J Hematol. 2003; 73:97–100. PMID: 12749010.

11. Colls BM. Guillain-Barre syndrome and hyponatremia. Intern Med J. 2003; 33:5–9. PMID: 12534871.
12. Nguyen MK, Rastogi A, Kurtz I. True hyponatremia secondary to intravenous immunoglobulin. Clin Exp Nephrol. 2006; 10:124–126. PMID: 16791398.

13. Davies AG. Inappropriate secretion of antidiuretic hormone in Guillain-Barre syndrome. Postgrad Med J. 1971; 47:651–653. PMID: 5158839.

14. Penney MD, Murphy D, Walters G. Resetting of osmoreceptor response as cause of hyponatraemia in acute idiopathic polyneuritis. Br Med J. 1979; ii:1474–1476. PMID: 526815.

15. Hochman MS, Kobetz SA, Handwerker JV. Inappropriate secretion of antidiuretic hormone associated with Guillain-Barre syndrome. Ann Neurol. 1982; 11:322–323. PMID: 7092187.
16. Kaneko K, Shioya T, Yabuta K. Inappropriate secretion of antidiuretic hormone and transient hypertension associated with Guillain-Barre syndrome. Pediatr Neurosci. 1989; 15:257–259. PMID: 2488953.
17. Hoffmann O, Reuter U, Schielke E, Weber JR. SIADH as the first symptom of Guillain-Barré syndrome. Neurology. 1999; 53:1365. PMID: 10522906.
18. Eisenman G, Rudin DO, Casby JU. Glass electrode for measuring sodium ion. Science. 1957; 126:831–834. PMID: 13467284.

19. Apple FS, Koch DD, Graves S, Ladenson JH. Relationship between direct-potentiometric and flame-photometric measurement of sodium in blood. Clin Chem. 1982; 28:1931–1935. PMID: 7127808.

20. Friedman SM, Wong SL, Walton JH. Glass electrode measurements of blood sodium and potassium in man. J Appl Physiol. 1963; 18:950–954. PMID: 14063266.

21. Moe OW, Fuster D. Clinical acid-base pathophysiology: disorders of plasma anion gap. Best Pract Res Clin Endocrinol Metab. 2003; 17:559–574. PMID: 14687589.

22. Albrink MJ, Hald PM, Man EB, Peters JP. Displacement of serum water by the lipids of serum. J Clin Invest. 1955; 34:1483–1488. PMID: 13263427.
23. Ladenson JH, Apple FS, Aguanno JJ, Koch DD. Sodium measurements in multiple myeloma: two techniques compared. Clin Chem. 1982; 28:2383–2386. PMID: 6814789.

24. Dimeski G, Mollee P, Carter A. Effects of hyperlipidemia on plasma sodium, potassium, and chloride measurements by an indirect ion-selective electrode measuring system. Clin Chem. 2006; 52:155–156. PMID: 16391336.

25. Levy GB. Determination of sodium with ion-selective electrodes. Clin Chem. 1981; 27:1435–1438. PMID: 7273405.

Fig. 1
Serum chemistries and osmolality pre- and post-IVIG infusion. Serum sodium and calculated osmolality significantly decreased and serum protein and viscosity significantly decreased 24 hr after the completion of IVIG therapy. From Reference 10.
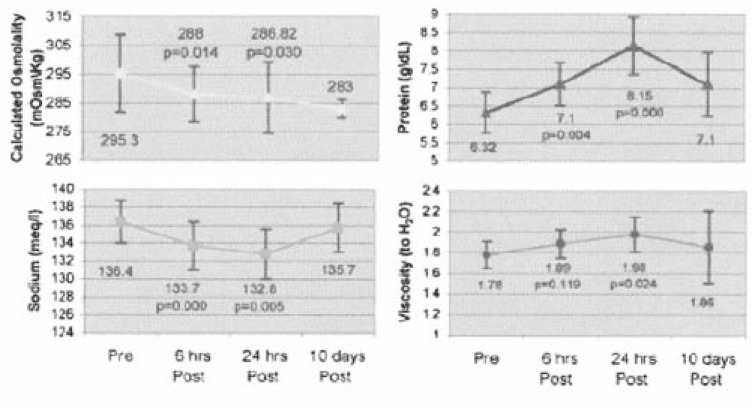
Fig. 2
Schematic representation of undiluted and diluted sampling techniques used by direct potentiometry and indirect potentiometry or flame photometry methods. From Reference 19.
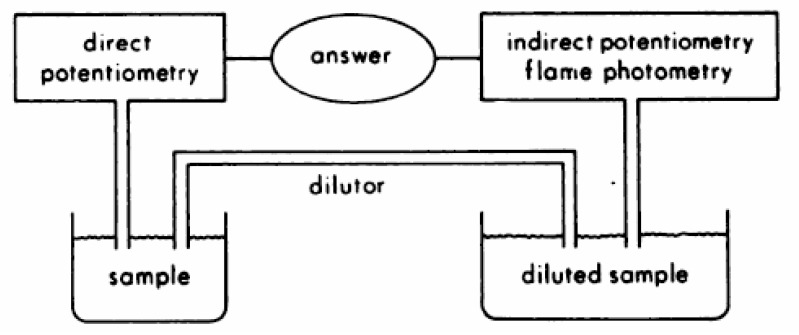
Fig. 3
Predicted Influence of water content on sodium measurements for 100 mmol/L NaCI solution. From Reference 19.
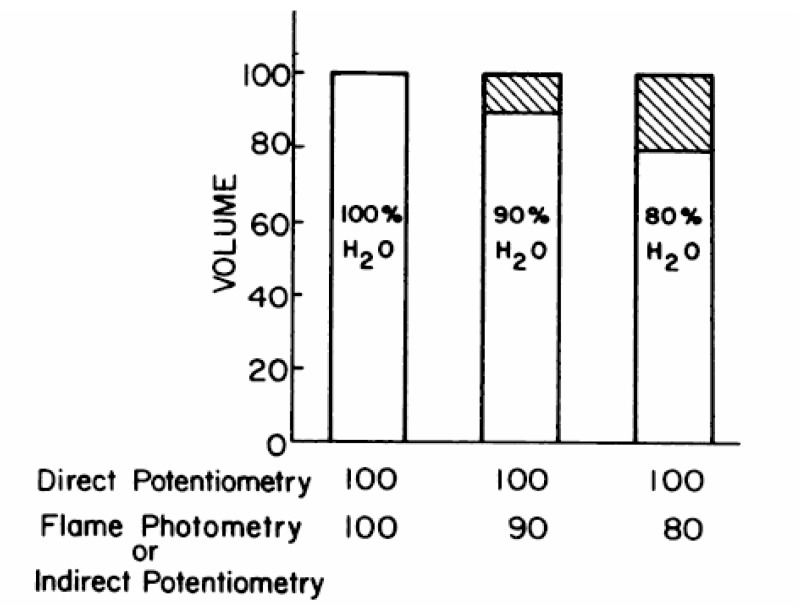
Fig. 4
Psesudohyponatremia from non-aqueous plasma volume. The use of ion-selective electrodes that measure Na+ activity theoretically rids the entity of pseudohyponatraemia from non-aqueous volumes in the plasma if the plasma is never diluted. However, automated aspirator/dilutors perpetuate this problem. The top panel depicts the situation in normal plasma. A 10×dilution of plasma with water dilutes [Na+] to 14 mEq/L and the back-calculation with a factor of 10 recapitulates the original plasma [Na+] of 140 mEq/L. The bottom panel depicts plasma with normal [Na+] of 140 but an excessive non-aqueous space. The aspirator will take an erroneously smaller amount of aqueous plasma and dilute it 10×with water, yielding a final [Na+] of 11.2 mEq/L. Back calculation with the same dilution factor gives a plasma [Na+] of 112 mEq/L. From Reference 21.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download