TO THE EDITOR: With approximately 7% of the worldwide population being carriers, hemoglobinopathies are the most common monogenic diseases [1]. The cumulative gene frequency of hemoglobinopathies in India is 4.2% [2]. Although liver involvement in the forms of intrahepatic cholestasis, hepatic crisis and cholelithiasis are common findings in patients with sickle cell disease [3], cholestasis is not a well-studied condition in non-transfusion dependent hemoglobinopathies. Herein, we report two cases of hemoglobin variants that presented with recurrent cholestasis and were managed with new therapeutic interventions.
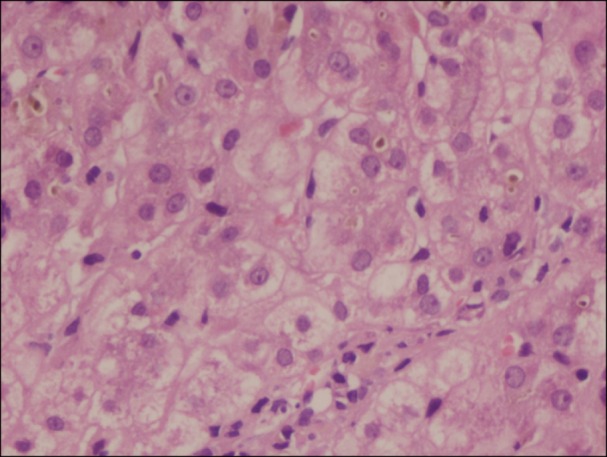
A 20-year-old Punjabi Sindhi man with no history of alcohol consumption presented with recurrent jaundice and abdominal pain. His past medical history is significant for occasional transfusion in last 3 years and taking alternative medicines intermittently for the last 5 years. During a previous episode, one month prior, he underwent an ERCP (Endoscopic retrograde cholangiopancreatography) with endoscopic papillotomy and balloon sweep for biliary pancreatitis. The laboratory results were: total bilirubin 13.5 mg/dL at presentation which progressed to 47 mg/dL over 3 days with direct bilirubin of 33.6 mg/dL, alanine transaminase (ALT) 147 IU/L, aspartate aminotransferase (AST) 162 IU/L, alkaline phosphatase (ALP) 200 IU/L and serum albumin of 3.5 g/dL. Differential diagnosis included viral hepatitis, biliary duct obstruction, autoimmune hepatitis, and Wilson's disease. Complete blood count results were: hemoglobin (Hb) level 10.0 g/dL, red blood cell (RBC) count 3.95×1012/L, mean corpuscular volume (MCV) 62.6 fL, mean corpuscular hemoglobin (MCH) 18.9 pg, mean corpuscular hemoglobin concentration (MCHC) 30.1 g/dL, red blood cell distribution width (RDW) 16.6%, white blood cell (WBC) 12,400/µL, and platelet within normal limits. HPLC (high performance liquid chromatography) revealed Hb A0 4.6%, Hb D-Punjab 84.5%, Hb A2 3.7%, and Hb F 3% suggestive of Hb D-β thalassemia. Family screening revealed that his mother was an Hb D carrier (Hb A0 52.5% Hb D Punjab 36.5% Hb A2 2.3%, Hb F 0.2%) and his father, a β thalassemia carrier (Hb A0 84.7, Hb A2 5.3%, Hb F 0.4%). Mutation analysis of the patient showed the presence of Hb D-Punjab; this was confirmed by polymerase chain reaction (PCR) amplification followed by digestion with EcoRI DNA analysis, which revealed nucleotide change at codon 121 (GAA-CAA) of the β-globin gene (known as Hb D Punjab). Concomitant analysis of the β-globin gene showed that the patient carried Hb D Punjab+IVS1-5 (G to C) mutation. Serum ferritin was 1,760 µg/L. Viral markers in the form of anti-hepatitis A virus (HAV) immunoglobulin M (IgM), hepatitis B surface antigen (HBsAg), anti-hepatitis A virus (HEV) IgM, cytomegalovirus (CMV) were nonreactive. Liver biopsy was done with patient's consent, which revealed acute hepatitis with cholestasis (drug related) (Fig. 1). As the patient was not on any alternative medicines for 3 weeks and had no clinical signs of liver failure when taking those drugs, all the criteria for drug-induced liver injury could not be fulfilled [4]. In light of his recurrent jaundice and absence of definitive evidence of drug-induced cholestasis, stains for MDR3 (multidrug resistance protein type 3) and BSEP (bile salt export pump) which may predispose individuals to drug-induced cholestasis were performed and were negative [5]. The liver dialysis (Prometheus) was performed twice to remove the excess bilirubin and protect (mainly) the brain and liver cells from its toxic effects. Serum bilirubin decreased to 6.8 mg/dL and other laboratory findings also returned to baseline. Patient was discharged in a stable condition and he started an oral chelation therapy. The patient was seen in follow up 4 weeks from discharge and is doing well.
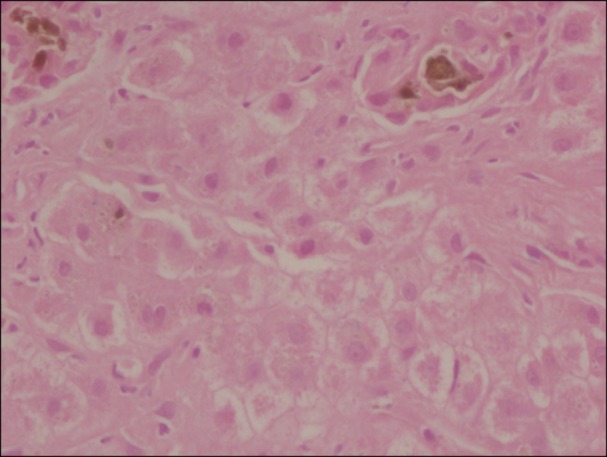
A 22-year-old woman from West Bengal presented with jaundice, abdominal pain, clay colored stools, and mild pruritus. Her past surgical history is significant for cholecystectomy at an age of 15 for jaundice. She has no history of blood transfusion. Abdominal examination revealed generalized tenderness, with a palpable liver 3 cm below the right costal margin and palpable spleen 10 cm below the left costal margin. The laboratory results were: total bilirubin 62.1 mg/dL, direct bilirubin of 41.1 mg/dL, ALT 161 IU/L, AST 190 IU/L, ALP 127 IU/L and serum albumin of 3.2 g/dL. Differential diagnosis of viral hepatitis, Wilson's disease, and autoimmune hepatitis were made. Complete blood count results were: Hb 9.0 g/dL, RBC count 3.82×1012/L, MCV 71.5 fL, MCH 23.1 pg, MCHC 32.3 g/dL, RDW 28.9%, WBC 18,300/µL and platelet within normal limit. Peripheral blood film showed moderate anisopoikilocytosis with microcytes, target cells, and moderate hypochromia with 14 NRBCs per 100 WBCs. Serum ferritin was 1,150 µg/L. Her HPLC results showed Hb E of 76.4%, Hb F 4.2%, Hb A0 3.8% and suggestive of Hb E disease. Both parents were found to be Hb E carriers. She was started on hepatoprotective measures - ursodeoxycholic acid and cholestyramine. Anti HAV IgM, HBsAg, Anti HEV IgM, and CMV were non-reactive. Antinuclear antibody (ANA) was positive. Liver biopsy revealed acute on chronic hepatitis with cholestasis (Fig. 2) and MDR3 and BSEP stains were negative. Her liver biopsy did not show features of iron overload by Perls' stain. Her total bilirubin persistently increased and eventually peaked at 72.6 mg/dL. Provisional diagnosis of autoimmune hepatitis was made. The patient underwent plasmapheresis for jaundice after five days of medical treatment, and was started on a trial of steroids after seven days. Her bilirubin decreased to 56 mg/dL after second session. Plasmapheresis was done safely and had no impact on ability of liver to regenerate. She underwent 3 sessions of plasmapheresis while liver transplant work up had started. She has improved clinically (total bilirubin 13.4 mg/dL) and is in follow up.
We have reported these cases to highlight new treatment modalities for cholestasis in hemoglobinopathies and stimulate the search for the etiopathogenesis. The prevalence of hemoglobinopathies varies with geographic locations and ethnic groups in India. Among the clinically important hemoglobinopathies (Hb S, Hb D, Hb E and beta thalassemia), hemoglobin E (Hb E) is mostly restricted to the North-eastern Indian states with an average allele frequency of 10.9% [6]. In a large multicenter study in India, HbD trait was more common among Sikhs (1.4%) and few individuals with HbD homozygous, HbD-β-thalassemia, HbD Iran trait, HbQ India trait, and Hb Lepore trait were also encountered at the different centers [7].
Heterozygous Hb D-Punjab is a clinically silent condition, but coinheritance of Hb D with Hb S or beta thalassemia produces clinically significant conditions like sickle cell anemia and chronic hemolytic anemia of moderate severity [8]. Recurrent jaundice is more commonly described in Hb SD due to intrahepatic cholestasis by sickling [9]. In the first case, the patient was diagnosed with Hb D-β thalassemia (with no features of iron overload) that required occasional transfusion. In this case, because of rapidly progressive hyperbilirubinemia, urgent intervention was required to reduce the morbidity. Of note, liver dialysis, which was used here, had decreased the bilirubin and related toxicity, is not described as a treatment modality in the literature for these patients. Papadopoulos et al. [10] described a case of a 28-year-old man with sickle cell disease, who presented with jaundice and abdominal pain, one month after hydroxyurea discontinuation with conjugated hyperbilirubinemia and choledocholithiasis. As jaundice had no signs of improvement, the patient was treated with single-pass albumin dialysis (SPAD). His laboratory values started to improve after one-and-a-half months of treatment. As high bilirubin levels predict a poor outcome in a number of prognostic models used for assessing the severity of acute (King's College criteria) or chronic (Child-Pugh score, MELD formula) liver disease, to prevent cellular toxicity, liver dialysis should be an option while waiting for liver transplantation [11].
Hb E presents with mild to moderate symptoms; most patients with the disease exhibit clinical symptoms by age ten. The most common presentation of Hb E disease is no or mild anemia, jaundice, fever, abdominal pain and gastrointestinal disturbances with or without splenomegaly [12]. In the second case, massive splenomegaly was due to associated chronic liver disease. In this case, cause of liver injury was most likely due to autoimmune hepatitis along with hemoglobinopathy. In literature, there are reports of using plasmapheresis as a therapeutic modality for treatment of cholestasis. Saritas et al. [13] used plasmapheresis as a therapeutic choice in patients with cholestasis, persistent pruritus and biochemical abnormalities despite treatment with ursodeoxycholic acid and glucocorticoids. Singer et al. [14] treated forty-nine patients with acute liver failure with a total of 243 therapeutic plasma exchanges (TPE). To best of our knowledge, no data is available on the use of plasmapheresis for cholestatic jaundice in patients with hemoglobinopathies.
Cholestatic liver diseases arise from impaired hepatobiliary production and excretion of bile, which cause bile constituents to enter the circulation [15]. In the cases discussed above, cause of cholestasis was not completely understood. The likely explanation of liver injury in these cases may be an underlying molecular defect that facilitated an acquired cause. Further research is needed to find the molecular mechanisms related to canalicular transporter genes in cholestasis of hemoglobinopathies as current treatment modalities are limited.
Cholestasis in hemoglobinopathies is a major challenge for both hematologist and hepatologist. The etiology and treatment of these cases have not been investigated yet in detail. Interestingly, our report has described liver dialysis and plasmapheresis to reduce the toxic effects of bilirubin. Until we find the molecular pathogenesis of severe cholestasis in these cases (if any), we should explore these techniques further to improve the outcome of these patients during their waiting periods for liver transplants.
References
1. Weatherall DJ. Hemoglobinopathies worldwide: present and future. Curr Mol Med. 2008; 8:592–599. PMID: 18991645.

2. Sarnaik SA. Thalassemia and related hemoglobinopathies. Indian J Pediatr. 2005; 72:319–324. PMID: 15876761.

3. Banerjee S, Owen C, Chopra S. Sickle cell hepatopathy. Hepatology. 2001; 33:1021–1028. PMID: 11343226.

4. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014; 109:950–966. PMID: 24935270.

5. Padda MS, Sanchez M, Akhtar AJ, Boyer JL. Drug-induced cholestasis. Hepatology. 2011; 53:1377–1387. PMID: 21480339.

6. Balgir RS. Genetic epidemiology of the three predominant abnormal hemoglobins in India. J Assoc Physicians India. 1996; 44:25–28. PMID: 8773089.
7. Mohanty D, Colah RB, Gorakshakar AC, et al. Prevalence of β-thalassemia and other haemoglobinopathies in six cities in India: a multicentre study. J Community Genet. 2013; 4:33–42. PMID: 23086467.

8. Ahmed M, Stuhrmann M, Bashawri L, Kühnau W, El-Harith EH. The beta-globin genotype E121Q/W15X (cd121GAA-->CAA/cd15TGG-->TGA) underlines Hb d/beta-(0) thalassaemia marked by domination of haemoglobin D. Ann Hematol. 2001; 80:629–633. PMID: 11757720.
9. Cawein MJ, Lappat EJ, Brangle RW, Farley CH. Hemoglobin S-D disease. Ann Intern Med. 1966; 64:62–70. PMID: 5900784.

10. Papadopoulos V, Karagianni A, Papageorgiou V, et al. Successful management of sickle cell intrahepatic cholestasis with combined use of exchange transfusion and single-pass albumin dialysis: A case report. Open J Blood Dis. 2013; 3:36–42.

11. Polson J, Lee WM. American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology. 2005; 41:1179–1197. PMID: 15841455.

12. Edison ES, Shaji RV, Srivastava A, Chandy M. Hyperbilirubinemia in homozygous HbE disease is associated with the UGT1A1 gene polymorphism. Hemoglobin. 2005; 29:189–195. PMID: 16114182.

13. Saritas U, Aydin B, Ustundag Y. Plasmapheresis and corticosteroid treatment for persistent jaundice after successful drainage of common bile duct stones by endoscopic retrograde cholangiography. World J Gastroenterol. 2007; 13:4152–4153. PMID: 17696241.

14. Singer AL, Olthoff KM, Kim H, Rand E, Zamir G, Shaked A. Role of plasmapheresis in the management of acute hepatic failure in children. Ann Surg. 2001; 234:418–424. PMID: 11524594.

15. Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010; 139:1481–1496. PMID: 20849855.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download