TO THE EDITOR: Prolongation of activated partial thromboplastin time (aPTT) is one of the common problems in the field of consultative hematology. When considering a patient with aPTT prolongation, other combined coagulation abnormalities should be identified first. If prolongation of prothrombin time (PT) or thrombocytopenia is also observed, possible causes such as liver disease or disseminated intravascular coagulation may be considered as one of the differential diagnoses. If a patient has no other coagulation abnormalities, including PT prolongation, thrombocytopenia, or decreased fibrinogen level, the patient can be considered to exhibit isolated aPTT prolongation. Isolated aPTT prolongation should be carefully examined, because hemophilia may also be present in this population [1]. In addition, confirmation through repeated tests is also needed, because various laboratory errors, including inadequate venous puncture, delayed analysis, incorrect preparation of plasma, and use of heparin, may cause isolated and transient aPTT prolongation.
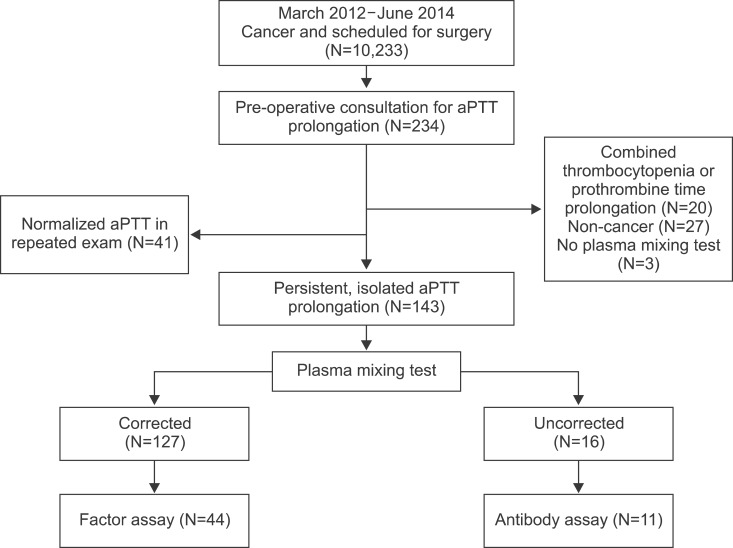
The plasma mixing test is the cornerstone test for the initial differential diagnosis of persistent and isolated aPTT prolongation; corrected cases suggest factor deficiency, while uncorrected cases suggest the presence of an inhibitor, such as lupus anticoagulant [2]. We examined the cause of isolated aPTT prolongation in cancer patients before cancer surgery.
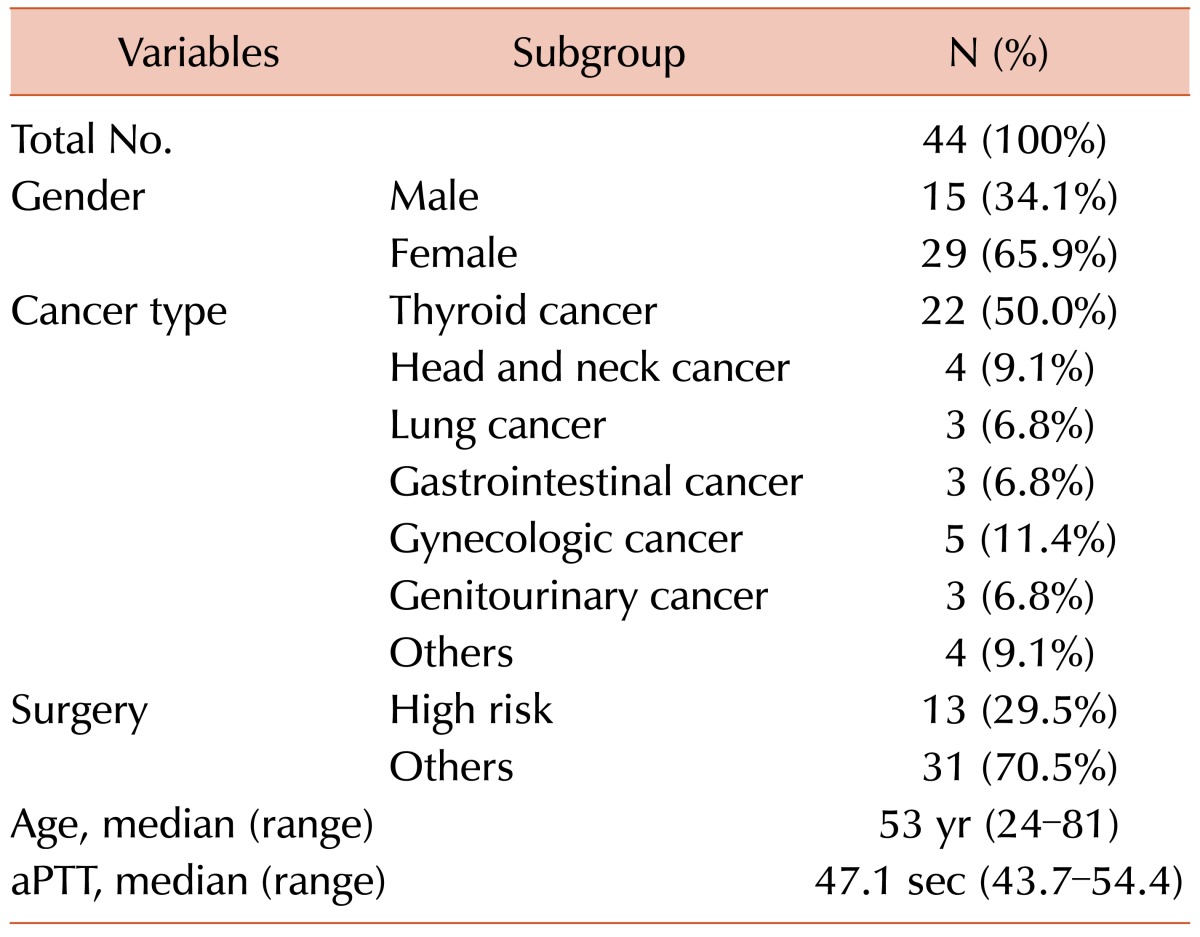
We found that most patients with isolated aPTT prolongation who were scheduled for cancer surgery had factor deficiency (88.8%), primarily factor XII (75.0%). Intrinsic factors of the coagulation pathway were measured in 44 patients (Fig. 1). Clinical data, including the history of bleeding tendency, type of surgery, and post-operative bleeding or thromboembolic outcomes, as well as laboratory results, including aPTT, change in hemoglobin level, activities of factors VIII, IX, XI, XII, von Willebrand factor antigen (vWF:Ag), and vWF ristocetin cofactor (vWF:RCo), were retrospectively reviewed. Bleeding risk associated with surgery was stratified as previously reported [3]. Surgeries associated with a high bleeding risk were as follows: coronary artery bypass, heart valve replacement surgery, intracranial or spinal surgery, aortic aneurysmal repair, peripheral artery bypass, other major vascular surgery, major orthopedic surgery (e.g., hip or knee replacement), reconstructive plastic surgery, major cancer surgery, prostate surgery, and bladder surgery. The median age was 53 years (range, 24-81 years), and 29 patients (65.9%) were female. Twenty-two patients (50.0%) were scheduled for surgery due to thyroid cancer, and 13 patients (29.5%) were scheduled to undergo an operation with a high risk of bleeding (Table 1).
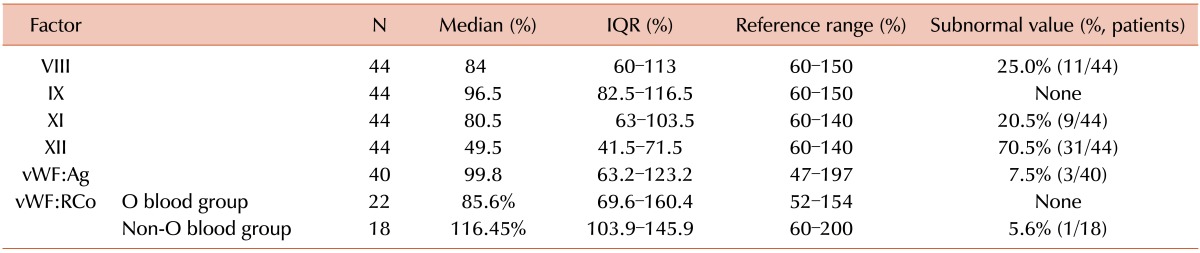
Among 44 patients, 75.0% had factor XII deficiency. Factor VIII and XI deficiency was detected in 25.0% and 20.5% of patients, respectively. In contrast, there was no patient with factor IX deficiency. Only a small percentage of patients showed vWF:Ag and vWF:RCo deficiency (7.5% and 2.5%, respectively; Table 2). The decrease in peripheral blood hemoglobin and hematocrit after surgery was -1.1±0.9 g/dL and -3.8±3.4% (mean±standard deviation), respectively. Post-operative events, including emergency operation and red blood cell (RBC) transfusion due to bleeding during or after surgery, occurred in only three patients. There was no thromboembolic event in 44 patients.
The first patient was a 53-year-old woman who had mild coagulation factor VIII deficiency (FVIII:C 41%) and who underwent left lobectomy of the thyroid and neck dissection due to thyroid cancer. She had experienced hematoma at the surgical site and underwent an emergency operation for hematoma evacuation on post-operative day 26. Unfortunately, we could not confirm the causal relationship between factor deficiency and the post-operative bleeding event in this case. The second patient was a 66-year-old woman with gynecological cancer. She had undergone major operations, including total colectomy, omentectomy, and peritonectomy and experienced post-operative disseminated intravascular coagulation. Accordingly, transfusion was needed in this patient. She had mild factor XII deficiency (FXII:C 39%) before surgery. The last patient was a 52-year-old man. He received bilateral T8-L3 posterior fixation due to spinal cord compression. He needed RBC transfusion due to intra-operative bleeding, although coagulation factor deficiency was not found. We could not detect a significant association between coagulation factor deficiency and post-operative bleeding in patients with cancer and isolated aPTT prolongation.
Our findings demonstrated that the main cause of isolated aPTT prolongation in patients with cancer might be a factor deficiency rather than the presence of an inhibitor such as lupus anticoagulant. In addition, the most commonly deficient factor was coagulation factor XII. In contrast, a previous study investigating the cause of isolated aPTT prolongation in general patients in an acute care setting demonstrated that the prevalent cause of aPTT prolongation was the presence of lupus anticoagulant [4]. When considered with our results, this suggests that the etiology of isolated aPTT prolongation might differ according to the disease subgroup. To our knowledge, the current study is the first to examine and report the cause of isolated aPTT prolongation and the frequency of factor deficiency of the intrinsic coagulation pathway in patients with cancer.
Factor XII deficiency, a rare congenital disorder, has sporadically been reported in case reports worldwide. Moreover, until the current report, there was only one report of three cases with genetically confirmed severe congenital factor XII deficiency in Korean patients [5]. Except for sporadic case reports, there have been only a few studies investigating factor XII deficiency in the clinical context. Factor XII deficiency is also known to be associated with thromboembolism as well as bleeding risk [6].
One Greek report showed that factor XII activity was significantly lower in women who experienced recurrent spontaneous abortion, while all normal controls had normal factor XII activity [7]. However, there was no peri-operative thromboembolic event in our study population. Furthermore, factor XII deficiency was not associated with post-operative bleeding events, though one female patient with mild factor XII deficiency received RBC transfusion after a major operation.
Although the degree of factor XII deficiency in our patients with cancer was mild and the definition of the lower limit of normal for factor XII is somewhat variable (54% to 70%) [89], we consider it valuable to report an unexpected high incidence of factor XII deficiency in patients with cancer and isolated aPTT prolongation.
Notes
References
1. Proctor RR, Rapaport SI. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol. 1961; 36:212–219. PMID: 13738153.

2. Kershaw G, Orellana D. Mixing tests: diagnostic aides in the investigation of prolonged prothrombin times and activated partial thromboplastin times. Semin Thromb Hemost. 2013; 39:283–290. PMID: 23457048.

3. Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133(6 Suppl):299S–339S. PMID: 18574269.
4. Chng WJ, Sum C, Kuperan P. Causes of isolated prolonged activated partial thromboplastin time in an acute care general hospital. Singapore Med J. 2005; 46:450–456. PMID: 16123828.
5. Kwon MJ, Kim HJ, Lee KO, Jung CW, Kim SH. Molecular genetic analysis of Korean patients with coagulation factor XII deficiency. Blood Coagul Fibrinolysis. 2010; 21:308–312. PMID: 20386432.

6. Zeerleder S, Schloesser M, Redondo M, et al. Reevaluation of the incidence of thromboembolic complications in congenital factor XII deficiency-a study on 73 subjects from 14 Swiss families. Thromb Haemost. 1999; 82:1240–1246. PMID: 10544906.
7. Dendrinos S, Deliveliotou A, Anastasiou A, Creatsas GK. Role of coagulation factor XII in unexplained recurrent abortions in the Greek population. J Reprod Med. 2014; 59:56–62. PMID: 24597288.
8. Kuhli C, Scharrer I, Koch F, Ohrloff C, Hattenbach LO. Factor XII deficiency: a thrombophilic risk factor for retinal vein occlusion. Am J Ophthalmol. 2004; 137:459–464. PMID: 15013868.

9. Braulke I, Pruggmayer M, Melloh P, Hinney B, Köstering H, Günther E. Factor XII (Hageman) deficiency in women with habitual abortion: new subpopulation of recurrent aborters? Fertil Steril. 1993; 59:98–101. PMID: 8419231.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download