1. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006; 47:2357–2363.
2. De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011; 89:754–765.
3. Gerdts E, Wachtell K, Omvik P, Otterstad JE, Oikarinen L, Boman K, Dahlöf B, Devereux RB. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: losartan intervention for end-point reduction in hypertension trial. Hypertension. 2007; 49:311–316.
4. Manning WJ, Gelfand EV. Left atrial size and postoperative atrial fibrillation: the volume of evidence suggests it is time to break an old habit. J Am Coll Cardiol. 2006; 48:787–789.
5. Laukkanen JA, Kurl S, Eränen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005; 165:1788–1793.
6. El Maghraby A, Hajar R. Giant left atrium: a review. Heart Views. 2012; 13:46–52.
7. Apostolakis E, Shuhaiber JH. The surgical management of giant left atrium. Eur J Cardiothorac Surg. 2008; 33:182–190.
8. Oh JK. Echocardiographic evaluation of morphological and hemodynamic significance of giant left atrium. An important lesson. Circulation. 1992; 86:328–330.
9. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16:233–270.
10. Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. Left atrial volume and geometry in healthy aging: the Cardiovascular Health Study. Circ Cardiovasc Imaging. 2009; 2:282–289.
11. Jiamsripong P, Honda T, Reuss CS, Hurst RT, Chaliki HP, Grill DE, Schneck SL, Tyler R, Khandheria BK, Lester SJ. Three methods for evaluation of left atrial volume. Eur J Echocardiogr. 2008; 9:351–355.
12. Grayburn PA, Weissman NJ, Zamorano JL. Quantitation of mitral regurgitation. Circulation. 2012; 126:2005–2017.
13. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129:e521–e643.
14. Plaschkes J, Borman JB, Merin G, Milwidsky H. Giant left atrium in rheumatic heart disease: a report of 18 cases treated by mitral valve replacement. Ann Surg. 1971; 174:194–201.
15. Johnson J, Danielson GK, MacVaugh H 3rd, Joyner CR. Plication of the giant left atrium at operation for severe mitral regurgitation. Surgery. 1967; 61:118–121.
16. Qian Y, Meng J, Tang H, Yang G, Deng Y, Wei D, Xiang B, Xiao X. Different structural remodelling in atrial fibrillation with different types of mitral valvular diseases. Europace. 2010; 12:371–377.
17. Wang L, Di Tullio MR, Beecham A, Slifer S, Rundek T, Homma S, Blanton SH, Sacco RL. A comprehensive genetic study on left atrium size in Caribbean Hispanics identifies potential candidate genes in 17p10. Circ Cardiovasc Genet. 2010; 3:386–392.
18. Matsuda H, Nakao M, Nohara H, Higami T, Mukohara N, Asada T, Ogawa K, Kawamura T. [The causes of prolonged postoperative respiratory care in mitral valve disease with a giant left atrium]. Kyobu Geka. 1990; 43:172–177.
19. Kawazoe K, Beppu S, Takahara Y, Nakajima N, Tanaka K, Ichihashi K, Fujita T, Manabe H. Surgical treatment of giant left atrium combined with mitral valvular disease. Plication procedure for reduction of compression to the left ventricle, bronchus, and pulmonary parenchyma. J Thorac Cardiovasc Surg. 1983; 85:885–892.
20. Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006; 47:1018–1023.
21. Mahabadi AA, Samy B, Seneviratne SK, Toepker MH, Bamberg F, Hoffmann U, Truong QA. Quantitative assessment of left atrial volume by electrocardiographic-gated contrast-enhanced multidetector computed tomography. J Cardiovasc Comput Tomogr. 2009; 3:80–87.

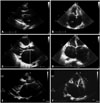
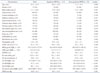
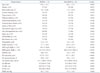
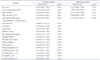




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download