Abstract
Background
Epicardial fat tissue has unique endocrine and paracrine functions that affect the cardiac autonomic system. The head-up tilt test (HUTT) is a simple non-invasive measurement that assesses autonomic nervous system dysfunction. We investigated the association between epicardial fat thickness (EFT) and autonomic neural tone, such as vagal tone.
Methods
A total of 797 consecutive patients (mean age 46.5 years, male: 45.7%) who underwent HUTT and echocardiography between March 2006 and June 2015 were enrolled. EFT was measured during the diastolic phase of the parasternal long axis view. We excluded patients with prior percutaneous coronary intervention, old age (* 70 years old), valvular heart disease, symptomatic arrhythmias and diabetes. We divided patients into two groups based on the HUTT (positive vs. negative).
Results
There were 329 patients (41.3%) with a negative HUTT result and 468 patients (58.7%) with a positive result. The HUTT-positive patients showed a significantly lower waist circumference, body mass index and systolic and diastolic blood pressure, although a significantly higher EFT as compared to the HUTT-negative patients (HUTT-positive, 5.69 ± 1.76 mm vs. HUTT-negative, 5.24 ± 1.60 mm; p < 0.001). EFT > 5.4 mm was associated with a positive HUTT result with 51.7% sensitivity and 63.8% specificity (p < 0.001) on receiving operator characteristic analysis. Multivariate Cox regression analysis revealed that EFT (hazard ratio: 1.02, 95% confidence interval: 1.01–1.30, p = 0.004) was an independent predictor of HUTT-positivity.
Vasovagal syncope (VVS) is the most common cause of postural syncope, and has a lifetime cumulative prevalence of 35%.1) The vasovagal decrease in heart rate or blood pressure (BP) usually leads to an increase in the reflex sympathetic stimulation of the ventricular myocardium, which in turn causes the activation of the ventricular vagal afferent C-fiber connecting to medullary vasomotor centers. The head-up tilt test (HUTT) is widely recognized as an effective technique for providing evidence of susceptibility to neurally-mediated syncope.2) Although the precise etiology and pathophysiological events underlying VVS are not fully understood,3) there is consensus that the autonomic nervous system is the final common pathway leading to syncope. Therefore, cardiovascular autonomic modulation may play a role in the occurrence of syncope in patients with HUTT-induced VVS.4)5)
Recently, there has been a great deal of interest on the potential role of epicardial adipose tissue (EAT) as a cardiovascular risk factor. Epicardial fat represents the visceral fat depot of the heart displaying high metabolic activity.6) A high amount of epicardial fat tissue has the capacity to produce several mediators with unique endocrine and paracrine functions that affect the cardiac autonomic system.6)7)8) Thus, we can hypothesize that cardiac autonomic function could explain the connection between epicardial fat thickness (EFT) and VVS. However, no previous studies have examined the association between HUTT-induced VVS and EFT as measured by echocardiography.
Therefore, the purpose of this study was to investigate the association between EFT and the HUTT in patients undergoing HUTT for the evaluation of unexplained syncope. Based on the key role of the autonomic nervous system in VVS, we hypothesized that increased EFT would reflect autonomic abnormalities that would help distinguish individuals with positive HUTT results from those with negative HUTT results.
This is retrospective, single-center study included a total of 855 patients with syncope or near syncope between March 2006 and June 2015, all patients underwent HUTT and echocardiography. Inclusion criteria were as follows: 18–70 years of age, normal renal function, and for women, a regular menstrual cycle. Exclusion criteria were: any systemic diseases such as significant liver disease, neurologic disorders or malignancy; secondary hypertension; valvular heart disease; symptomatic arrhythmia; a positive treadmill test; a history of heart failure; a history of acute coronary syndrome; a history of myocardial infarction or any revascularization procedure. And we also excluded all type 2 diabetes mellitus patients to avoid effect of diabetes in autonomic cardiac modulation and VVS. Demographic characteristics recorded at the first visit included age, sex, height, weight, current medications, smoking history and past medical history. Blood was drawn for the measurement of total serum cholesterol, triglycerides, high-density lipoprotein (HDL) and low-density lipoprotein cholesterol, blood glucose, creatinine, uric acid, and high sensitivity C-reactive protein. Body mass index (BMI) was calculated as the ratio of dry weight in kilograms to height squared (in square meters). This study was approved by the institutional review board. All patients were required to provide written informed consent to participate.
The HUTT was performed after overnight fasting and during sinus rhythm, usually between 8:00 am and 12:00 pm. All cardioactive and vasoactive drugs were withdrawn at least 7 days before. Informed consent was obtained from all participants. The patient was stabilized in the supine position for 5 min and tilted at 60° for 20 min to induce syncope. If syncope was not induced, the patient was given 2.5 mg of sublingual nitroglycerin spray at a 60° tilt. Twelve-lead ECG tracings and BP were measured every 2.5 min throughout the HUTT. If syncope or hypotension (systolic BP < 80 mm Hg) occurred, the patient was moved into the Trendelenburg position and the test was promptly terminated. A positive response to the HUTT indicated the development of syncope or presyncope in association with hypotension, bradycardia, or both. We divided the patients into two groups based on HUTT results (positive vs. negative). The patients who experienced syncope during the test were divided into three groups based on the presence of bradycardia, hypotension, or both during the onset of syncope. A vasodepressor response was defined as a decrease in systolic BP to < 60 mm Hg without a decrease in heart rate during symptoms. A cardio-inhibitory response was defined as an abrupt decrease in heart rate by ≥ 20% without any antecedent decrease in systolic BP. A mixed response was defined as a concurrent decrease in systolic BP to < 60 mm Hg and a decrease in heart rate by ≥ 20% compared to these values 4 min before the onset of symptoms.
We performed echocardiogram all of the patients to differentiate the structural heart disease causing syncope such as aortic stenosis, hypertrophic cardiomyopathy, left ventricular outflow tract obstruction. Echocardiogram was performed immediately before HUTT. Standard 2-dimensional echocardiography was performed on all participants while lying in the left lateral decubitus position using a 3.5-MHz transducer (Philips iE33, Philips Medical Systems, Bothell, WA, USA). The echocardiographers were blinded to patient information. Measurements of the thickness of the interventricular septum and posterior wall, the diameter of the left ventricle (LV) cavity, and the LV mass index were performed according to the criteria outlined by the American Society of Echocardiography.9) Echocardiographic assessments of EFT, defined as the echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium, were measured perpendicularly from the free wall of the right ventricle at end-systole in three cardiac cycles according to the method we previously described.10) Because one critical issue in EFT measurement is inconsistency of measurement location, the mean EFT was averaged from images from the parasternal long axis, parasternal short axis and apical 4-chamber view. The intra- and inter-observer variability of the EFT was 3.3% and 5.8%, respectively.
Statistical analyses were performed with the commercially available computer program SPSS 18.0 for Windows (IBM Corp., Armonk, NY, USA). Data were presented as mean ± standard deviation for continuous variables and their percentages (%) for categorical data. The Mann-Whitney U test was used for continuous variables and the chi-square test was used for categorical data. The normality of the data was tested using the Kolmogorov-Smirnov test. Parameter differences between groups were evaluated using an independent Student's t-test for normally distributed variables or the Kruskal-Wallis test for non-normally distributed variables. Relationships between variables were examined with Pearson correlation coefficients. The cutoff value of EFT for predicting a positive HUTT with corresponding sensitivity and specificity was estimated by receiving operator characteristic (ROC) curve analysis. Multivariate logistic regression models for a positive HUTT were built to determine which variables were independently associated with this status. A two-tailed p < 0.05 was considered statistically significant.
A total of 797 consecutive patients (mean age 46.5 years, male: 45.7%) were analyzed and their clinical features according to HUTT result are shown in Table 1. There were 329 patients (41.3%) with negative HUTT results and 468 patients (58.7%) with positive HUTT results. There were no differences between subjects with positive and negative HUTT results with respect to age and heart rate (Table 1). However, HUTT-positive patients were more likely to be female and have a significantly lower waist circumference, BMI, and systolic and diastolic BPs. When analyzing only male patients, both waist circumference and BMI was lower in HUTT-positive patients and higher EFT than HUTT-negative group (Supplementary Table 1). When analyzing only female patients, there was no significant difference of waist circumference and BMI between groups (Supplementary Table 2). According to the hemodynamic responses accompanying the syncope, 468 HUTT-positive participants were classified as follows: 62 vasodepressive responses, 107 cardio-inhibitory responses, and 299 mixed responses.
There were no significant differences in cardiac chamber size between groups with the exception of the left atrium, ejection fraction, and mitral inflow patterns (Table 2).
However, EFT was significantly higher in HUTT-positive patients compared to HUTT-negative patients (HUTT-positive, 5.69 ± 1.76 mm vs. HUTT-negative, 5.24 ± 1.60 mm; p < 0.001) (Fig. 1A). Furthermore, we estimated EFT values among the three hemodynamic types of syncope, and found that there were no significant differences among the groups with respect to EFT (vasodepressive responses, 5.6 ± 1.5 mm; cardio-inhibitory response, 5.8 ± 1.9 mm; and mixed responses, 5.7 ± 1.8 mm; p = 0.669) (Fig. 1B).
On the ROC analysis, EFT > 5.4 mm was associated with a positive HUTT result with 51.7% sensitivity and 63.8% specificity [area under curve: 0.578, 95% confidence interval (CI) = 0.54–0.61, p < 0.001] (Fig. 2). Univariate logistic regression analysis showed that a positive response was associated with female sex, left atrial diameter, HDL cholesterol, waist circumference, systolic BP and EFT [hazard ratio (HR): 1.02, 95% CI: 1.01–1.03, p < 0.001]. Furthermore, multivariate logistic regression analysis revealed that EFT (HR: 1.02, 95% CI: 1.01–1.03, p = 0.004) was an independent predictor of a positive HUTT result (Table 3) after adjusting for variables with significant univariate impact. When we performed subgroup analysis for the multivariate regression according to the use or non-use of medications, in particular beta blockers, the results did not differ.
To the best of our knowledge, this is the first study investigating the association between EFT and HUTT. The most relevant findings obtained from this study are as follows: 1) epicardial fat was thicker in patients with a positive HUTT result, 2) multiple logistic regression analyses showed that an increased EFT was an independent predictor of positive HUTT in patients with unexplained syncope. Our findings suggest a possible link between epicardial fat and autonomic dysregulation in HUTT-positive VVS.
Although VVS is a common clinical event, its pathophysiological mechanism has not yet been fully elucidated. The syncopal symptoms of VVS appear to be associated with pathological autonomic cardiac modulation.11)12) The neurally-mediated syncopal syndromes encompass a number of apparently related disturbances of reflex cardiovascular control characterized by transient inappropriate bradycardia and/or vasodilation of various arterial and venous beds. VVS results from simultaneous enhancement of parasympathetic nervous system (vagal) tone and abrupt withdrawal of sympathetic nervous system tone.13)14)15)16) In addition, it was postulated that there is a ‘ mismatch' between the neural signal to the skeletal muscle vasculature and the release or availability of noradrenaline.17) One previous study showed that an imbalance in myocardial autonomic nerve function plays an important role in inducing VVS.4) HUTT is a wellvalidated and effective technique for the evaluation of vasovagal reactions, which may be responsible for syncopal episodes.18) Thus, it is conceivable that patients with HUTT-positive VVS present with abnormalities of autonomic circulatory control.
Increased plasma fatty acid levels may stimulate the cardiac autonomic nervous system through an increase in plasma catecholamine concentrations. EAT is anatomic storage of fat with endocrine, paracrine, and vasocrine effects on the myocardium. 19) These effects are due to the lack of a fibrous fascial layer impeding diffusion of free fatty acids and adipokines between the tissue and the underlying vessel wall and myocardium.20)21) Moreover, EAT contains abundant ganglionic plexi, which interact with the extrinsic sympathetic and parasympathetic nervous system to modulate the cardiac autonomic system.22)23) Thus, patients with a large amount of EAT may have increased intrinsic adrenergic and cholinergic nerves.20) A recent study showed that altered sympathovagal balance, as detected by disturbed heart rate variability and heart rate turbulence, was observed in patients with higher EAT thickness, and this influence was independently correlated with EAT thickness.24) Interestingly, in our study, HUTT-positive group showed lower BMI and waist circumstance than HUTT-negative group. Although epicardial fat is known to be associated with metabolic syndrome, hypervagotonic activity is more likely to be affected in patients with VVS. There may be possible mechanism that people who are obese of having high body mass can respond and adapt better to situation of fainting of vasovagal event than thin people. There have been approved that a low weight and low BMI has been found to be a risk factor of VVS during transfusion.25) So we think EAT is not simple reflecting parameter of visceral fat. It may have own pathophysiology and element to affects vagal activity.
Consequently, there may be a possible correlation between EFT as assessed by echocardiography and HUTT-positivity in patients with neurally-mediated syncope. The principal finding of our study is that patients with VVS with a positive tilt test also had increased EFT. In addition, there was a significant and independent interaction between EFT and HUTT-positivity, which can be explained by the fact that the EAT contains rich ganglionic plexi that have both adrenergic and cholinergic nerves. Interestingly, the increase in the EFT was not dependent upon the hemodynamic type of syncope. To the best of our knowledge, this is the first study to evaluate the relationship between EFT as an indicator of autonomic nervous system disturbances and a positive HUTT result in patients with VVS. Although this conclusion cannot be drawn with certainty, we speculate that the discordance between the autonomic nervous system and increased intrinsic cardiac autonomic tone may lead to a deterioration of baroreceptor-cardiac reflex sensitivity in subjects with greater EAT thickness.
Consequently, there may be a possible correlation between EFT as assessed by echocardiography and HUTT-positivity in patients with neurally-mediated syncope. The principal finding of our study is that patients with VVS with a positive tilt test also had increased EFT. In addition, there was a significant and independent interaction between EFT and HUTT-positivity, which can be explained by the fact that the EAT contains rich ganglionic plexi that have both adrenergic and cholinergic nerves. Interestingly, the increase in the EFT was not dependent upon the hemodynamic type of syncope. To the best of our knowledge, this is the first study to evaluate the relationship between EFT as an indicator of autonomic nervous system disturbances and a positive HUTT result in patients with VVS. Although this conclusion cannot be drawn with certainty, we speculate that the discordance between the autonomic nervous system and increased intrinsic cardiac autonomic tone may lead to a deterioration of baroreceptor-cardiac reflex sensitivity in subjects with greater EAT thickness.
Our study has several limitations. First, to decrease the effect of ischemic symptoms after the termination of the exercise test, we excluded patients with a history of heart failure, revascularization, or a positive result on an exercise test. However, the presence of coronary artery disease was excluded using only exercise testing, and further stress imaging modalities were not used. Second, this study is retrospective and was performed at a single tertiary care center, it is possible that there were biases with respect to patient referral and population sampling. Third, the correlation power between EFT and HUTT result was weak. Forth, we didn't analyzed linear correlations between EFT and changes of BP or HR during HUTT. But when we estimated EFT values among the three hemodynamic types of syncope, there were no significant differences among the groups with respect to EFT. Finally, EFT can be affected by metabolic syndrome, raising the possibility of an association between metabolic syndrome and EFT.
In conclusion, EFT, an indicator of cardiac autonomic activity, was increased in patients with HUTT-positive VVS, in whom abnormal parasympathetic reactivation occurs. EFT was an independent predictor of a positive HUTT. Our findings suggest a possible link between epicardial fat and autonomic dysregulation in HUTT-positive patients with VVS. The association between EFT and adverse cardiovascular outcomes in HUTTpositive patients with VVS needs to be investigated in further detail through future research.
Figures and Tables
Fig. 1
Comparison of epicardial fat thickness (EFT) according to the head-up tilt test (HUTT). EFT was significantly higher in HUTT-positive patients compared to HUTT-negative patients (A). However, there were no significant differences among the three hemodynamic types of syncope groups with respect to EFT (B).

Fig. 2
On the receiving operator characteristic analysis, epicardial fat thickness > 5.4 mm was associated with a positive head-up tilt test (HUTT) result with 51.7% sensitivity and 63.8% specificity (area under curve: 0.578, 95% confidence interval = 0.54–0.61, p < 0.001).

Table 2
Comparison of echocardiographic parameters according to the HUTT result
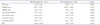
All values are presented as the mean ± SD. HUTT: head-up tilt test, EFT: epicardial fat thickness, LVEDD: left ventricular end-diastolic diameter, LVESD: left ventricular end-systolic diameter, EF: ejection fraction, LA: left atrial, E: peak early diastolic mitral filling velocity, Ea: mitral annular velocity, A: peak late diastolic mitral filling velocity
References
1. Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, van Dijk N. Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35-60 years. J Cardiovasc Electrophysiol. 2006; 17:1172–1176.
2. Kapoor WN, Smith MA, Miller NL. Upright tilt testing in evaluating syncope: a comprehensive literature review. Am J Med. 1994; 97:78–88.
3. Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983; 1:90–102.
4. Kochiadakis G, Marketou M, Koukouraki S, Parthenakis F, Chlouverakis G, Karkavitsas N, Vardas P. Cardiac autonomic disturbances in patients with vasovagal syndrome: comparison between iodine-123-metaiodobenzylguanidine myocardial scintigraphy and heart rate variability. Europace. 2012; 14:1352–1358.
5. Shinohara T, Ebata Y, Ayabe R, Fukui A, Okada N, Yufu K, Nakagawa M, Takahashi N. Cardiac autonomic dysfunction in patients with head-up tilt test-induced vasovagal syncope. Pacing Clin Electrophysiol. 2014; 37:1694–1701.
6. Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O'Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009; 30:850–856.
7. Sengul C, Duman D. The association of epicardial fat thickness with blunted heart rate recovery in patients with metabolic syndrome. Tohoku J Exp Med. 2011; 224:257–262.
8. Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008; 117:605–613.
9. Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987; 59:956–960.
10. Shim IK, Cho KI, Kim HS, Heo JH, Cha TJ. Impact of gender on the association of epicardial fat thickness, obesity, and circadian blood pressure pattern in hypertensive patients. J Diabetes Res. 2015; 2015:924539.
11. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982; 6:287–291.
12. Gupta BN, Thames MD. Behavior of left ventricular mechanoreceptors with myelinated and nonmyelinated afferent vagal fibers in cats. Circ Res. 1983; 52:291–301.
13. Abi-Samra F, Maloney JD, Fouad-Tarazi FM, Castle LW. The usefulness of head-up tilt testing and hemodynamic investigations in the workup of syncope of unknown origin. Pacing Clin Electrophysiol. 1988; 11:1202–1214.
14. Lagi A, Tamburini C, Fattorini L, Cencetti S. Autonomic control of heart rate variability in vasovagal syncope: a study of the nighttime period in 24-hour recordings. Clin Auton Res. 1999; 9:179–183.
15. Jardine DL, Ikram H, Frampton CM, Frethey R, Bennett SI, Crozier IG. Autonomic control of vasovagal syncope. Am J Physiol. 1998; 274(6 Pt 2):H2110–H2115.
16. Jardine DL, Ikram H, Crozier IG. Autonomic control of asystolic vasovagal syncope. Heart. 1996; 75:528–530.
17. Vaddadi G, Esler MD, Dawood T, Lambert E. Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur Heart J. 2010; 31:2027–2033.
18. Tercedor L, Díaz JF, Aguado MJ, Moreno E, Molina E, Alvarez M, Ramírez JA, Pérez de la Cruz JM, Azpitarte J. [The tilt-table test in assessing syncope of unknown origin: do differences exist between children and adults?]. Rev Esp Cardiol. 1999; 52:189–195.
19. Gaborit B, Venteclef N, Ancel P, Pelloux V, Gariboldi V, Leprince P, Amour J, Hatem SN, Jouve E, Dutour A, Clément K. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc Res. 2015; 108:62–73.
20. Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005; 2:536–543.
21. Sarin S, Wenger C, Marwaha A, Qureshi A, Go BD, Woomert CA, Clark K, Nassef LA, Shirani J. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol. 2008; 102:767–771.
22. Chen PS, Turker I. Epicardial adipose tissue and neural mechanisms of atrial fibrillation. Circ Arrhythm Electrophysiol. 2012; 5:618–620.
23. Ardell JL. The cardiac neuronal hierarchy and susceptibility to arrhythmias. Heart Rhythm. 2011; 8:590–591.
24. Balcioğlu AS, Çiçek D, Akinci S, Eldem HO, Bal UA, Okyay K, Müderrisoğlu H. Arrhythmogenic evidence for epicardial adipose tissue: heart rate variability and turbulence are influenced by epicardial fat thickness. Pacing Clin Electrophysiol. 2015; 38:99–106.
25. Takanashi M, Odajima T, Aota S, Sudoh M, Yamaga Y, Ono Y, Yoshinaga K, Motoji T, Matsuzaki K, Satake M, Sugimori H, Nakajima K. Risk factor analysis of vasovagal reaction from blood donation. Transfus Apher Sci. 2012; 47:319–325.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.4250/jcu.2017.25.2.57.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download