Abstract
Background
Methods
Results
Figures and Tables
Fig. 1
Screenshot of the Mitral-Valve-Quantification software showing the volume-rendered 3D data set (bottom right) as well as the three cut planes used to improve the visualization of the mitral valve. Ao: aorta, A: anterior, P: posterior, AL: anterolateral, PM: posteromedial.
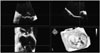
Fig. 2
Three-dimensional reconstruction of the mitral valve, from which several parameters were automatically calculated. From top to bottom, left to right: anterolateral to posteromedial diameter of annulus; anterior to posterior diameter of annulus; mitral annular height, defined as the height of the bounding box of the mitral valve in the atrial-ventricular direction; maximal prolapse height; maximal tenting height; area of annulus in projection plane; exposed area of anterior leaflet; exposed area of posterior leaflet; perimeter of annulus; aortic orifice to mitral plane angle; length of coaptation in projection plane; exposed length of A2; exposed length of P2; volume of leaflet prolapse; volume of the leaflets tent; angle of anterior leaflet; nonplanar angle of leaflets; angle of posterior leaflet; annular height to commissural width ratio. Ao: aorta, A: anterior, P: posterior, AL: anterolateral, PM: posteromedial.
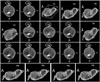
Fig. 3
Scatter diagram with Pearson's correlation of the annular height/BSA by RT3D-TEE and the degree of LAVI decrease between echocardiography obtained at baseline and at least 6 months postoperatively. LAVI: left atrial volume index, BSA: body surface area, RT3D-TEE: real-time 3D transesophageal echocardiography.

Table 1
Baseline characteristics of patients with severe mitral regurgitation and decreased or normal annular height/BSA
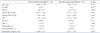
Data are listed as mean value (percentage). The p value denotes statistical significance when comparing the non-remodeling and remodeling groups. *p < 0.05 on Student's t-test (continuous variables) or chi-square test (categorical variables). BSA: body surface area, BP: blood pressure, HR: heart rate, HTN: hypertension, DM: diabetes mellitus
Table 2
Preoperative echocardiographic parameters in severe mitral regurgitation with decreased or normal annular height/BSA
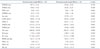
Data are listed as mean value (percentage). The p value denotes statistical significance when comparing the non-remodeling and remodeling groups. p < 0.05 on Student's t-test (continuous variables) or chi-square test (categorical variables). BSA: body surface area, LVEDD: left ventricular end-diastolic dimension, LVESD: left ventricular end-systolic dimension, IVSd: diastolic interventricular septum thickness, LVPWd: diastolic left ventricular posterior wall thickness, Ao: aorta, LAVI: left atrial volume index, LVEF: left ventricular ejection fraction, LVEDV: left ventricular end-diastolic volume, LVESV: left ventricular end-systolic volume, DT: deceleration time, RVSP: right ventricular systolic pressure, PISA: proximal isovelocity surface area, ERO: effective regurgitant orifice, RV: regurgitant volume
Table 3
Postoperative echocardiographic parameters in severe MR with decreased or normal annular height/BSA
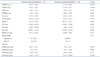
Data are listed as mean value (percentage). The p value denotes statistical significance when comparing non-remodeling and remodeling groups. *p < 0.05 on Student's t-test (continuous variables) or chi-square test (categorical variables). BSA: body surface area, LVEDD : left ventricular end-diastolic dimension, LVESD: left ventricular end-systolic dimension, IVSd: diastolic interventricular septum thickness, LVPWd: diastolic left ventricular posterior wall thickness, Ao: aorta, LAVI: left atrial volume index, LVEF: left ventricular ejection fraction, DT: deceleration time, RVSP: right ventricular systolic pressure, MR: mitral regurgitation, pre-post: difference between echocardiography parameters at baseline and at least 6 months post-operation
Table 4
Preoperative RT3D-TEE parameters in severe mitral regurgitation with decreased or normal annular height/BSA at baseline on 3D echocardiography
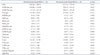
Data are listed as mean value (percentage). The p value denotes statistical significance when comparing non-remodeling and remodeling groups. *p < 0.05 on Student's t-test (continuous variables), chi-square test (categorical variables). RT3D-TEE: real-time 3D transesophageal echocardiography, BSA: body surface area, A2D: area of annulus in projection plane, A3DE Ant_pre: exposed area of anterior leaflet, A3DE Post_pre: exposed area of the posterior leaflet, C3D_pre: perimeter of the annulus, DALPm_pre: anterolateral to posteromedial diameter of the annulus = intercommissural diameter, DAP_pre: anterior to posterior diameter of the annulus, Hprol_pre: maximal prolapse height, Htent_pre: maximal tenting height, L2DAlPm_pre: length of coaptation in the projection plane, L3DE A2_pre: exposed length of A2, L3DE P2_pre: exposed length of P2, Vprol_pre: volume of leaflet prolapse, Vtent_pre: volume of the leaflet tent, θ_pre: aortic orifice to mitral plane angle, θ Ant_pre: angle of the anterior leaflet, θ NPA_pre: non-planar angle of leaflets, θ Post_pre: angle of the posterior leaflet, AHCWR: annular height to commissural width ratio
Table 5
Immediate postoperative RT3D-TEE parameters in severe mitral regurgitation with decreased or normal annular height/BSA
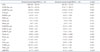
Data are listed as mean value (percentage). The p value denotes statistical significance when comparing non-remodeling and remodeling groups. *p < 0.05 on Student's t-test (continuous variables), chi-square test (categorical variables). RT3D-TEE: real-time 3D transesophageal echocardiography, BSA: body surface area, A2D: area of the annulus in the projection plane, A3DE Ant_pre: exposed area of the anterior leaflet, A3DE Post_pre: exposed area of the posterior leaflet, C3D_pre: perimeter of the annulus, DALPm_pre: anterolateral to posteromedial diameter of the annulus = intercommissural diameter, DAP_pre: anterior to posterior diameter of the annulus, Hprol_pre: maximal prolapse height, Htent_pre: maximal tenting height, L2DAlPm_pre: length of coaptation in the projection plane, L3DE A2_pre: exposed length of A2, L3DE P2_pre: exposed length of P2, Vprol_pre: volume of leaflet prolapse, Vtent_pre: volume of the leaflet tent, θ_pre: Aortic orifice to mitral plane angle, θ Ant_pre: angle of the anterior leaflet, θ NPA_pre: non-planar angle of leaflets, θ Post_pre: angle of the posterior leaflet, AHCWR: annular height to commissural width ratio
Table 6
Univariate linear analysis and multivariate logistic regression analysis of determinants of postoperative LAVI remodeling
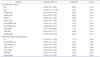
LAVI: left atrial volume index, BSA: body surface area, LVEDD: left ventricular end-diastolic dimension, LVESD: left ventricular end-systolic dimension, LVEF: left ventricular ejection fraction, H_pre: annulus height, AHCWR: annular height to commissural width ratio, MR: mitral regurgitation, CI: confidence interval




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download