Abstract
In patients with end-stage left ventricular (LV) heart failure who receive LV assist device (LVAD) implantation, right ventricular (RV) failure represents a possible critical complication that heavily affects morbidity and mortality. Several clinical, laboratory, hemodynamic, and echocardiographic variables have been found to be associated with RV failure occurrence after surgery. Different models and risk scores have been proposed, with poor results. No accordance has ever been reached about RV pre-operative evaluation, and time has come to introduce a standardized systematic protocol for LVAD suitability assessment according to RV function. We analyzed imaging parameters associated with LVAD implantation-related RV failure, in order to identify the minimum number for pre-operative reliable prediction of post-operative RV failure. A few echocardiographic parameters have been identified as the most reliable, or promising, and reproducible tools in this field: free-wall RV longitudinal strain, RV fractional area change, RV sphericity index, and RV ejection fraction with 3D-echocardiography. We propose the Systematic LVAD Implant Eligibility with Non-invasive Assessment protocol–the SIENA protocol–as a new and simple way of pre-operative evaluation of patients candidates to LVAD implantation.
Treatment of end-stage left ventricular (LV) heart failure (HF) is a constantly updating health issue. Although a fundamental resource, heart transplantation remains a limited tool, widely insufficient to satisfy the demand of refractory HF patients treatment. LV assist devices (LVAD) have been progressively shifting from bridge to transplantation therapy alone to an increasing use as destination therapy for not suitable for transplantation-patients.1)2) Even if this fact represents a thrilling advancement in HF treatment, it opens the question of suitability to LVAD implantation. In fact, failing hearts may vary widely even at end-stage disease. Current data on morbidity and mortality in patients underwent to LVAD implantation confirm such assumption.3)
More specifically, a focus on right ventricle needs to be open. Right ventricular (RV) systolic function has a strong ability to stratify prognosis in patients with HF and a coexistent RV dysfunction is associated with poorer exercise capacity and reduced survival in these patients.4)5)6)7)8)9) RV systolic function is the best recognized factor on whom the outcome of LVAD patients depends.10)11)12)13) Nonetheless, according to different definitions, 6–44% of patients undergo RV failure after LVAD surgery.14)15) The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) defines RV failure as need of an RV assist device (RVAD) or requirement of inhaled nitric oxide or inotropic therapy for > 1 week any time after LVAD implantation in the presence of symptoms and signs of persistent RV dysfunction, such as central venous pressure > 18 mm Hg with a cardiac index < 2.3 L/min per square meter in the absence of elevated left atrial or pulmonary capillary wedge pressure (> 18 mm Hg), cardiac tamponade, ventricular arrhythmias, or pneumothorax.1) It is therefore crucial to evaluate RV morphology and, above all, function prior to confirm the eligibility of any patients to LVAD. It appears, however, equally important to monitor the right ventricle during the last days before device implantation, in order to minimize both long-term and short-term RV dysfunction. Transthoracic echocardiography is a reliable, versatile and bedside-available tool for this kind of evaluation.
Factors determining RV output and function include RV preload, afterload, and contractility. Implant of LVADs inevitably affects all such factors, possibly leading to acute RV dysfunction. RV preload increases as a result of increased cardiac index–which can augment up to 100%.16) In addition, perioperative period constitutes an adjunctive stress since a substantial amount of fluids and blood products are often administered to patients. This acute increase in preload leads to overstretching of cardiac myofibrils beyond the point of optimal contractility based on Frank-Starling principle, and thus to decreased RV stroke volume. Furthermore, increase in RV preload may lead to RV annular dilatation and tricuspid regurgitation, adding further load to RV demand. Since RV function and, secondly, structure in HF patients is strictly dependent on the effect of LV dysfunction itself,17) a consistent number of patients with terminal LV dysfunction unfortunately have coexistent at least initial RV dysfunction. Fleeting or weak right ventricles may not tolerate the new hemodynamic equilibrium following LVAD implantation and, consequently, fail.
In recent years, many studies have tried to find predictors or to develop models and scores aimed to foretell RV failure risk after LVAD implantation.11)12)18)19)20)21)22)23)24)25)26)27)28)29)30)31)32) A plethora of clinical, laboratory, hemodynamic, and echocardiographic variables have been found to be associated with RV failure or need of RVAD implantation. Cardiac index, RV stroke work index (RVSWI), vasopressor requirement, serum creatinine, bilirubin or aspartate aminotransferase, blood pressure, preoperative low pulmonary arterial pressure, tricuspid incompetence, RV short/long axis ratio, tricuspid annular motion, and RV longitudinal strain (RVLS) are just some examples. It is evident how poor accordance exist in this field, not only about which variables are linked to RV failure, but also about a more basic point: the focus on RV response capability towards increased preload, increased cardiac output and continuous aortic flow that follow LVAD implantation. There is a strong rationale for considering RV contractility as the key point.
The aim of such models is to exclude the eligibility to LVAD through an accurate patients selection. The matter is not to identify overt biventricular failure but to intercept those patients with slight, initial, subclinical RV dysfunction who are particularly likely to develop frank RV failure after LVAD, thus encountering a poorer prognosis. This knowledge may pave the way to further development of differentiated standardized protocols of peri-operatory management for patients at major risk of RV failure after surgery, which may be a far better solution rather than pre-operative or, even worst, post-operative shift to biventricular assist device. Transthoracic echocardiography provides the most reliable and, very importantly, soon and easily repeatable parameters for RV evaluation, allowing a tight follow-up in perioperative period as well as on long-term period.
Physicians' attention should be shifted from “what to do if RV failure occurs after LVAD implantation” to “how can we avoid RV failure to occur after LVAD implantation.” Aim of this paper is to provide a concise review of RV echocardiographic indexes of function in the setting of assessing patients eligibility to LVAD implantation, and to propose a protocol to be standardized as a routine tool in patients evaluation in order to reduce morbidity and mortality among these patients.
Despite RV ultrasound imaging has been challenging and, as a consequence, neglected for a long time, there are several parameters which reliably describe RV morphology and function.
Normal RV function is highly dependent on longitudinal shortening. Tricuspid annular plane systolic excursion (TAPSE) measured through M-mode is a widely used index of RV function (Fig. 1). In the initial validation study, TAPSE correlated strongly with radionuclide angiography, with low interobserver variability.33) Nevertheless, TAPSE is a regional, linear parameter for a complex structure and is dependent on loading conditions and insonation angle. Therefore, it is not uncommon to under- or over-estimate RV systolic function according to TAPSE, especially in failing hearts. In a study of 750 patients with a variety of cardiac conditions, TAPSE yielded high specificity but low sensitivity to distinguish abnormal from normal subjects.34) Although TAPSE has been reported to predict RV failure,23) further studies in LVAD patients did not confirm this finding.35)36)37)
Tissue Doppler imaging (TDI) is an attractive alternative to TAPSE because myocardial velocities are easy to obtain and reproduce (Fig. 2). Systolic velocity (S') of the tricuspid annulus reflects longitudinal RV function. However, velocities depend on insonation angle and loading conditions. Also, translational motion of the heart and tethering by adjacent diseased myocardial segments can produce velocities that are not representative of the performance of the interrogated segment. In a study of 68 recipients with LVAD, systolic tricuspid annular velocity did not predict RV failure.38)
Among imaging techniques, speckle tracking echocardiography (STE) has recently been applied to the study of RV function. It allows an objective and quantitative evaluation of global and regional myocardial function, independent of the angle of insonation and from cardiac translational movements (Fig. 3).39)
In a retrospective study of 117 patients undergoing LVAD implantation, free-wall RVLS by velocity vector imaging predicted RV failure with 76% specificity and 68% sensitivity at a cutoff of -9.6%.10) In another study of 68 patients undergoing elective LVAD surgery, RVLS by speckle tracking was significantly impaired preoperatively (-12.6 ± 3.3% vs. -16.2 ± 4.3%; p < 0.001) in 24 patients (35.3%) who experienced RV failure by 14 days.38)
In other studies, it has been demonstrated that in patients with advanced systolic HF referred for heart transplantation, STE analysis of RV deformation correlates well with RVSWI, an invasive measurement of RV systolic function, providing a better estimation of RV systolic performance than other traditional parameters.30)35) In particular, close negative correlations between global RVLS and free-wall RVLS with the RVSWI were found (r = -0.75 and -0.82, respectively; both p < 0.0001).35) Furthermore, free-wall RVLS demonstrated the highest diagnostic accuracy [area under the receiver operating characteristic curve (AUC): 0.90] and good sensitivity and specificity of 92% and 86%, respectively, to predict depressed RVSWI using a cutoff value of less than -11.8%.35) In the same study, tricuspid S' on TDI and TAPSE were not significantly correlated with RVSWI (r = 0.14 and 0.06; respectively).
Moreover, it has been demonstrated that a low free-wall RVLS has the highest predictive value for post-operatory RV failure in accordance with poor RVSWI in patients underwent to LVAD implantation (AUC: 0.93).40) Finally, in a recent perspective study on 98 patients with systolic HF referred for heart transplantation, during a mean follow-up of 1.5 ± 0.9 years, free-wall RVLS and global RVLS were independently predictive (both p < 0.0001) of combined outcomes of cardiovascular death, hospitalization for acute HF, heart transplantation, intra-aortic balloon pump implantation, and ventricular assist device implantation.41) The overall performance for the prediction of cardiovascular events was greatest for free-wall RVLS (AUC: free-wall RVLS: 0.87; global RVLS: 0.67).
RV fractional area change (RVFAC) is a feasible, quantitative technique which allows to estimate RV systolic function. Defined as (end-diastolic area - end-systolic area) / end-diastolic area × 100, it is a measure of RV systolic function which has been shown to correlate with RV ejection fraction (RVEF) by magnetic resonance imaging (MRI).42)43) In the above mentioned study, RVFAC was independently predictive of combined cardiovascular outcomes (cardiovascular death, hospitalization for acute HF, heart transplantation, intra-aortic balloon pump implantation and ventricular assist device implantation) in advanced HF patients referred for heart transplantation (p < 0.0001; AUC: 0.60).41) In a retrospective study of patients implanted with LVAD, RVFAC was significantly lower in the group of patients who experienced RV failure than in the group without RV failure (24% vs. 30%; p = 0.04).36) However, RVFAC did not predict RV failure in another larger study.44) Technical issues make this index less reproducible than RVLS, such as heavy RV trabeculation and pacemaker or defibrillator-related artifacts in patients with advanced HF. In one study, a > 10% reduction in RVFAC at 1 month was associated with worse quality of life and poor exercise capacity in patients with an LVAD.45)
RVEF is a powerful index of RV systolic performance and is generally calculated through cardiac MRI.46) Despite that, many end-stage HF patients are still foreclosed to MRI, being receivers of implanted cardioverter defibrillator or cardiac resynchronization therapy (CRT) devices, that might not be MRI compatible. Three-dimensional (3D) echocardiography has been extensively validated against cardiac MRI.47)48) 3D-echocardiography has become widespread and relatively easy to use. Recently, it has been proposed as an effective alternative to MRI for RVEF calculation in patients with LV dysfunction,49) allowing to overcome technical issues on MRI and providing reliable and objective measurement of RV systolic function. Growing experience and technology development have allowed the comparison of 3D-derived RV volumes and EF with other echo and MRI well established functional parameters.50)51) Specifically, it is notable to underline that ultimate software releases for the analysis of RV function have allowed to significantly ameliorate feasibility and reproducibility since a higher level of semi-automaticity has been introduced.50) Nonetheless, ECG-gated MRI might be limited for RVEF evaluation in patients with significant variability of cardiac cycles duration, such as in atrial fibrillation which is highly prevalent in end-stage HF patients: single-beat 3D-echocardiography might allow to overcome such question.52) Recently, Nagata et al.53) found that RVEF assessed through 3D-echocardiography was independently associated with cardiac outcomes in patients with diverse backgrounds, after determination of methodology accuracy against MRI. For all these reasons, we believe that 3D echocardiography is already a valuable tool for functional evaluation of the right ventricle.
RV index of myocardial performance (RIMP) is a functional global index of both systolic and diastolic RV function. It is calculated as the ratio of the isovolumic contraction and relaxation times to the ejection time. A higher ratio means that a large proportion of each cardiac cycle is spent increasing and decreasing pressure without performing stroke work, indicating a worse ventricular performance.54) In patients with advanced HF selected to receive CRT, abnormal RIMP demonstrated to be associated with adverse outcome compared to normal RIMP (0.83 vs. 0.69, p = 0.004) and each 0.1 unit increase in RIMP was associated with a 16% increased risk (95% confidence interval: 8–26).55) Analogous association was also found in patients with moderate chronic HF.56)
The RV response to chronic volume and/or pressure overload is dilation. Dilation means a loss of triangular shape in a two-dimensional 4-chamber view imaging, and a tendency towards sphericization. Minor dimensions–i.e., basal and mid diameters–of the right ventricle progressively increase, whereas longitudinal dimension is generally poorly modified (Fig. 4).57) RV sphericity index (RVSI), expressed as the ratio between RV midventricular and longitudinal diameters, is an indicator of RV remodeling that has recently been studied in patients with LV HF. In a cohort of 62 LV HF patients with increased pulmonary arterial hypertension, an increase in RVSI in one-year follow-up predicted clinical deterioration with good sensitivity and specificity (respectively, 70% and 62%, AUC: 0.649).58) RVSI was also increased in a cohort of patients undergoing heart transplant for end-stage HF.59)
Echocardiography provides a potentially complete evaluation of RV function in the setting of advanced HF. The echocardiographic indices used in this context are resumed in Table 1, with possible benefits and disadvantages in clinical usage for each of them.
Since RV failure can complicate LVAD implantation, pre-surgery RV analysis should be performed systematically in order to minimize morbidity and mortality of patients treated with LVAD. A systematic pre-operative RV study should be aimed at stratifying the risk of RV post-operative dysfunction. For this aim we will to propose the SIENA protocol, an echocardiographic scoring system, in order to emphasize the need of a systematic ultrasound evaluation of RV function prior to LVAD surgery.
In this review of echocardiographic parameters of RV function, free-wall RVLS, RVFAC, RVEF with 3D-echocardiography and RVSI have emerged as the most promising or best predictive indexes of RV dysfunction after LVAD implantation.
These indices were therefore introduced in our model for the prediction of post-LVAD RV failure. Table 2 summarizes their reference values and our proposal of a systematic protocol that should eventually be investigated in a multicenter, longitudinal study. Fig. 5 supplies a visual abstract of the SIENA protocol.
As a provisional analysis, based on single studies analyzing the single parameters for that aim, a high level of specificity for these parameters appears clear. Waiting a longitudinal study, we propose to give one point for each positive parameter and to identify a higher risk of RV failure for patients with more than one (SIENA score > 1). Subsequently, if data are confirmed, we could use this score as an additional tool to identify patients at higher risk that would be worth of a more intensive evaluation and management.
RV failure after LVAD implantation still remains a huge unsolved problem and constitutes an unacceptable source of morbidity and mortality. Time has come to find an accordance about risk stratification in pre-operatory evaluation of patients undergoing LVAD. Assessment of RV capability to react to the new hemodynamic setting due to LVAD is a key point, and transthoracic echocardiography provides the best indexes of RV function. We think that RV evaluation in LVAD-candidate patients should be a routine analysis in every center. In particular, we propose the Systematic LVAD Implant Eligibility Non-invasive Assessment protocol, the SIENA protocol, to be studied as a routine program of suitability evaluation before LVAD implantation focused on RV pre-operatory function. Such protocol would be part of a more global clinical evaluation of patients, being based on transthoracic echocardiographic variables which include: free-wall RVLS, RVFAC, RVSI, and RVEF with 3D-echocardiography. Feasibility, quick availability and reproducibility appear as the highlights of this protocol. A multicenter prospective study needs to be performed in order to eventually establish its potentially powerful role.
Figures and Tables
Fig. 1
Tricuspid annular plane systolic excursion (TAPSE) in a patient with end-stage left ventricular heart failure. In this case TAPSE is depressed (13 mm). Image acquired with a high quality sonogram (Vivid 7, GE General Electric, Horten, Norway) with 2.5 MHz transducer.
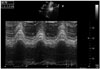
Fig. 2
Tissue Doppler imaging of the right ventricle with pulsed Doppler sample volume placed in the tricuspid annulus in a patient with end-stage left ventricular heart failure. In this case S' is depressed (0.07 m/s). Image acquired with a high quality sonogram (Vivid 7, GE General Electric, Horten, Norway) with 2.5 MHz transducer.
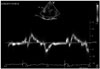
Fig. 3
Free-wall right ventricular longitudinal strain (RVLS) with speckle tracking echocardiography in a patient with end-stage left ventricular heart failure. In this case, free-wall RVLS is normal (> -16%). Image acquired with a high quality sonogram (Vivid 7, GE General Electric, Horten, Norway) with 2.5 MHz transducer and a semi-automatic 2D strain software (EchoPAC, GE General Electric).
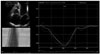
Fig. 4
Right ventricular sphericity index in a patient with end-stage left ventricular heart failure (0.66). Image acquired with a high quality sonogram (Vivid 7, GE General Electric, Horten, Norway) with 2.5 MHz transducer.
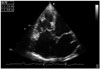
Fig. 5
A visual summary of the echocardiographic parameters included in the SIENA protocol. RVSI: right ventricular sphericity index, RVFAC: right ventricular fractional area change, RVLS: right ventricular longitudinal strain, RVEF: right ventricular ejection fraction.
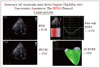
Table 1
Benefits and disadvatages of echocardiographic indices of RV function in evaluating eligibility to LVAD implantation
HF: heart failure, RV: right ventricular, LVAD: left ventricular assist device, MRI: magnetic resonance imaging, RIMP: RV index of myocardial performance, RVSI: RV sphericity index, RVFAC: RV fractional area change, RVLS: RV longitudinal strain, RVEF: RV ejection fraction, TAPSE: tricuspid annular plane systolic excursion, TDI: tissue Doppler imaging, AUC: area under the receiver operating characteristic curve, S': systolic velocity
References
1. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Miller MA, Baldwin JT, Young JB. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014; 33:555–564.
2. Kyo S, Minami T, Nishimura T, Gojo S, Ono M. New era for therapeutic strategy for heart failure: destination therapy by left ventricular assist device. J Cardiol. 2012; 59:101–109.
3. Katz MR, Dickinson MG, Raval NY, Slater JP, Dean DA, Zeevi GR, Horn EM, Salemi A. Outcomes of patients implanted with a left ventricular assist device at nontransplant mechanical circulatory support centers. Am J Cardiol. 2015; 115:1254–1259.
4. Gavazzi A, Berzuini C, Campana C, Inserra C, Ponzetta M, Sebastiani R, Ghio S, Recusani F. Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant. 1997; 16:774–785.
5. de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998; 32:948–954.
6. Ghio S, Tavazzi L. Right ventricular dysfunction in advanced heart failure. Ital Heart J. 2005; 6:852–855.
7. Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995; 25:1143–1153.
8. Meluzin J, Spinarová L, Hude P, Krejcí J, Dusek L, Vítovec J, Panovsky R. Combined right ventricular systolic and diastolic dysfunction represents a strong determinant of poor prognosis in patients with symptomatic heart failure. Int J Cardiol. 2005; 105:164–173.
9. Lindqvist P, Calcutteea A, Henein M. Echocardiography in the assessment of right heart function. Eur J Echocardiogr. 2008; 9:225–234.
10. Maeder MT, Leet A, Ross A, Esmore D, Kaye DM. Changes in right ventricular function during continuous-flow left ventricular assist device support [corrected]. J Heart Lung Transplant. 2009; 28:360–366.
11. Fitzpatrick JR 3rd, Frederick JR, Hsu VM, Kozin ED, O'Hara ML, Howell E, Dougherty D, McCormick RC, Laporte CA, Cohen JE, Southerland KW, Howard JL, Jessup ML, Morris RJ, Acker MA, Woo YJ. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. 2008; 27:1286–1292.
12. Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008; 51:2163–2172.
13. Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation. 2010; 122:173–183.
14. Kavarana MN, Pessin-Minsley MS, Urtecho J, Catanese KA, Flannery M, Oz MC, Naka Y. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg. 2002; 73:745–750.
15. Koprivanac M, Kelava M, Sirić F, Cruz VB, Moazami N, Mihaljević T. Predictors of right ventricular failure after left ventricular assist device implantation. Croat Med J. 2014; 55:587–595.
16. Farrar DJ, Hill JD, Pennington DG, McBride LR, Holman WL, Kormos RL, Esmore D, Gray LA Jr, Seifert PE, Schoettle GP, Moore CH, Hendry PJ, Bhayana JN. Preoperative and postoperative comparison of patients with univentricular and biventricular support with the thoratec ventricular assist device as a bridge to cardiac transplantation. J Thorac Cardiovasc Surg. 1997; 113:202–209.
17. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006; 114:1883–1891.
18. Grant AD, Smedira NG, Starling RC, Marwick TH. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol. 2012; 60:521–528.
19. Kukucka M, Stepanenko A, Potapov E, Krabatsch T, Redlin M, Mladenow A, Kuppe H, Hetzer R, Habazettl H. Right-to-left ventricular end-diastolic diameter ratio and prediction of right ventricular failure with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2011; 30:64–69.
20. Fukamachi K, McCarthy PM, Smedira NG, Vargo RL, Starling RC, Young JB. Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thorac Surg. 1999; 68:2181–2184.
21. Ochiai Y, McCarthy PM, Smedira NG, Banbury MK, Navia JL, Feng J, Hsu AP, Yeager ML, Buda T, Hoercher KJ, Howard MW, Takagaki M, Doi K, Fukamachi K. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. 2002; 106:12 Suppl 1. I198–I202.
22. Potapov EV, Stepanenko A, Dandel M, Kukucka M, Lehmkuhl HB, Weng Y, Hennig F, Krabatsch T, Hetzer R. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant. 2008; 27:1275–1281.
23. Puwanant S, Hamilton KK, Klodell CT, Hill JA, Schofield RS, Cleeton TS, Pauly DF, Aranda JM Jr. Tricuspid annular motion as a predictor of severe right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant. 2008; 27:1102–1107.
24. Drakos SG, Janicki L, Horne BD, Kfoury AG, Reid BB, Clayson S, Horton K, Haddad F, Li DY, Renlund DG, Fisher PW. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2010; 105:1030–1035.
25. Hennig F, Stepanenko AV, Lehmkuhl HB, Kukucka M, Dandel M, Krabatsch T, Hetzer R, Potapov EV. Neurohumoral and inflammatory markers for prediction of right ventricular failure after implantation of a left ventricular assist device. Gen Thorac Cardiovasc Surg. 2011; 59:19–24.
26. Topilsky Y, Oh JK, Shah DK, Boilson BA, Schirger JA, Kushwaha SS, Pereira NL, Park SJ. Echocardiographic predictors of adverse outcomes after continuous left ventricular assist device implantation. JACC Cardiovasc Imaging. 2011; 4:211–222.
27. Wang Y, Simon MA, Bonde P, Harris BU, Teuteberg JJ, Kormos RL, Antaki JF. Decision tree for adjuvant right ventricular support in patients receiving a left ventricular assist device. J Heart Lung Transplant. 2012; 31:140–149.
28. Atluri P, Goldstone AB, Fairman AS, MacArthur JW, Shudo Y, Cohen JE, Acker AL, Hiesinger W, Howard JL, Acker MA, Woo YJ. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann Thorac Surg. 2013; 96:857–863. discussion 863-4.
29. Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, Farrar DJ. HeartMate II Clinical Investigators. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010; 139:1316–1324.
30. Cameli M, Bernazzali S, Lisi M, Tsioulpas C, Croccia MG, Lisi G, Maccherini M, Mondillo S. Right ventricular longitudinal strain and right ventricular stroke work index in patients with severe heart failure: left ventricular assist device suitability for transplant candidates. Transplant Proc. 2012; 44:2013–2015.
31. Kaczorowski DJ, Woo YJ. Who needs an RVAD in addition to an LVAD? Cardiol Clin. 2011; 29:599–605.
32. Pettinari M, Jacobs S, Rega F, Verbelen T, Droogne W, Meyns B. Are right ventricular risk scores useful? Eur J Cardiothorac Surg. 2012; 42:621–626.
33. Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984; 107:526–531.
34. Tamborini G, Pepi M, Galli CA, Maltagliati A, Celeste F, Muratori M, Rezvanieh S, Veglia F. Feasibility and accuracy of a routine echocardiographic assessment of right ventricular function. Int J Cardiol. 2007; 115:86–89.
35. Cameli M, Lisi M, Righini FM, Tsioulpas C, Bernazzali S, Maccherini M, Sani G, Ballo P, Galderisi M, Mondillo S. Right ventricular longitudinal strain correlates well with right ventricular stroke work index in patients with advanced heart failure referred for heart transplantation. J Card Fail. 2012; 18:208–215.
36. Raina A, Seetha Rammohan HR, Gertz ZM, Rame JE, Woo YJ, Kirkpatrick JN. Postoperative right ventricular failure after left ventricular assist device placement is predicted by preoperative echocardiographic structural, hemodynamic, and functional parameters. J Card Fail. 2013; 19:16–24.
37. Kukucka M, Stepanenko A, Potapov E, Krabatsch T, Kuppe H, Habazettl H. Impact of tricuspid valve annulus dilation on mid-term survival after implantation of a left ventricular assist device. J Heart Lung Transplant. 2012; 31:967–971.
38. Kato TS, Jiang J, Schulze PC, Jorde U, Uriel N, Kitada S, Takayama H, Naka Y, Mancini D, Gillam L, Homma S, Farr M. Serial echocardiography using tissue Doppler and speckle tracking imaging to monitor right ventricular failure before and after left ventricular assist device surgery. JACC Heart Fail. 2013; 1:216–222.
39. Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zacà V, Ballo P, D'Andrea A, Muraru D, Losi M, Agricola E, D'Errico A, Buralli S, Sciomer S, Nistri S, Badano L. Echocardiography Study Group Of The Italian Society Of Cardiology (Rome, Italy). Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med. 2011; 30:71–83.
40. Cameli M, Lisi M, Righini FM, Focardi M, Lunghetti S, Bernazzali S, Marchetti L, Biagioli B, Galderisi M, Maccherini M, Sani G, Mondillo S. Speckle tracking echocardiography as a new technique to evaluate right ventricular function in patients with left ventricular assist device therapy. J Heart Lung Transplant. 2013; 32:424–430.
41. Cameli M, Righini FM, Lisi M, Bennati E, Navarri R, Lunghetti S, Padeletti M, Cameli P, Tsioulpas C, Bernazzali S, Maccherini M, Sani G, Henein M, Mondillo S. Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am J Cardiol. 2013; 112:1778–1784.
42. Lai WW, Gauvreau K, Rivera ES, Saleeb S, Powell AJ, Geva T. Accuracy of guideline recommendations for two-dimensional quantification of the right ventricle by echocardiography. Int J Cardiovasc Imaging. 2008; 24:691–698.
43. Anavekar NS, Gerson D, Skali H, Kwong RY, Yucel EK, Solomon SD. Two-dimensional assessment of right ventricular function: an echocardiographic-MRI correlative study. Echocardiography. 2007; 24:452–456.
44. Kato TS, Farr M, Schulze PC, Maurer M, Shahzad K, Iwata S, Homma S, Jorde U, Takayama H, Naka Y, Gillam L, Mancini D. Usefulness of two-dimensional echocardiographic parameters of the left side of the heart to predict right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2012; 109:246–251.
45. Lam KM, Ennis S, O'Driscoll G, Solis JM, Macgillivray T, Picard MH. Observations from non-invasive measures of right heart hemodynamics in left ventricular assist device patients. J Am Soc Echocardiogr. 2009; 22:1055–1062.
46. Alfakih K, Reid S, Jones T, Sivananthan M. Assessment of ventricular function and mass by cardiac magnetic resonance imaging. Eur Radiol. 2004; 14:1813–1822.
47. Shimada YJ, Shiota M, Siegel RJ, Shiota T. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: a meta-analysis study. J Am Soc Echocardiogr. 2010; 23:943–953.
48. Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, Bartolles R, Baumann R, Schummers G, Lang RM, Nesser HJ. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc Imaging. 2010; 3:10–18.
49. Kim J, Cohen SB, Atalay MK, Maslow AD, Poppas A. Quantitative assessment of right ventricular volumes and ejection fraction in patients with left ventricular systolic dysfunction by real time three-dimensional echocardiography versus cardiac magnetic resonance imaging. Echocardiography. 2015; 32:805–812.
50. Muraru D, Spadotto V, Cecchetto A, Romeo G, Aruta P, Ermacora D, Jenei C, Cucchini U, Iliceto S, Badano LP. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur Heart J Cardiovasc Imaging. 2016; 17:1279–1289.
51. Ostenfeld E, Flachskampf FA. Assessment of right ventricular volumes and ejection fraction by echocardiography: from geometric approximations to realistic shapes. Echo Res Pract. 2015; 2:R1–R11.
52. Park JB, Lee SP, Lee JH, Yoon YE, Park EA, Kim HK, Lee W, Kim YJ, Cho GY, Sohn DW. Quantification of right ventricular volume and function using single-beat three-dimensional echocardiography: a validation study with cardiac magnetic resonance. J Am Soc Echocardiogr. 2016; 29:392–401.
53. Nagata Y, Wu VC, Kado Y, Otani K, Lin FC, Otsuji Y, Negishi K, Takeuchi M. Prognostic value of right ventricular ejection fraction assessed by transthoracic 3D echocardiography. Circ Cardiovasc Imaging. 2017; 10:e005384.
54. Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol. 1995; 26:357–366.
55. Field ME, Solomon SD, Lewis EF, Kramer DB, Baughman KL, Stevenson LW, Tedrow UB. Right ventricular dysfunction and adverse outcome in patients with advanced heart failure. J Card Fail. 2006; 12:616–620.
56. Vizzardi E, D'Aloia A, Bordonali T, Bugatti S, Piovanelli B, Bonadei I, Quinzani F, Rovetta R, Vaccari A, Curnis A, Dei Cas L. Long-term prognostic value of the right ventricular myocardial performance index compared to other indexes of right ventricular function in patients with moderate chronic heart failure. Echocardiography. 2012; 29:773–778.
57. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008; 117:1436–1448.
58. Grapsa J, Gibbs JS, Cabrita IZ, Watson GF, Pavlopoulos H, Dawson D, Gin-Sing W, Howard LS, Nihoyannopoulos P. The association of clinical outcome with right atrial and ventricular remodelling in patients with pulmonary arterial hypertension: study with real-time three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012; 13:666–672.
59. Lisi M, Cameli M, Righini FM, Malandrino A, Tacchini D, Focardi M, Tsioulpas C, Bernazzali S, Tanganelli P, Maccherini M, Mondillo S, Henein MY. RV longitudinal deformation correlates with myocardial fibrosis in patients with end-stage heart failure. JACC Cardiovasc Imaging. 2015; 8:514–522.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download