Abstract
Background
Bronchopulmonary dysplasia (BPD) may result in chronic pulmonary artery hypertension and right ventricular (RV) dysfunction. Various echocardiographic assessments of RV dysfunction have been used to determine whether echocardiographic measurements of premature infants with BPD could provide sensitive measures of RV function that correlates with BPD severity.
Methods
Twenty-eight control subjects without BPD (non BPD group), 28 patients with mild BPD, 11 patients with moderate BPD, and six patients with severe BPD underwent echocardiograms with standard measurement such as ejection fraction by M-mode, tricuspid regurgitation pressure gradient, myocardial performance index (MPI) derived from pulse Doppler, and tissue Doppler imaging (TDI) measurements. BPD severity was classified by the NICHD/NHLBI/ORD workshop rating scale. Twenty-eight control subjects without BPD (non BPD group), 28 patients with mild BPD, 11 patients with moderate BPD, and six patients with severe BPD underwent echocardiograms with standard measurement such as ejection fraction by M-mode, tricuspid regurgitation pressure gradient, myocardial performance index (MPI) derived from pulse Doppler, and TDI measurements. BPD severity was classified by the NICHD/NHLBI/ORD workshop rating scale.
Results
None of the standard echocardiographic findings was significantly different between the control group and BPD groups. However, mean septal TDI-MPI of the severe BPD group (0.68 ± 0.06) was significantly (p < 0.01) higher than that of the non-BPD (0.58 ± 0.10) or the mild BPD group (0.59 ± 0.12). In addition, mean RV TDI-MPI of the severe BPD group (0.71 ± 0.13) was significantly (p < 0.05) higher than that of the non-BPD group (0.56 ± 0.08) or the mild BPD group (0.60 ± 0.125). Linear regression showed a good correlation between the severity of BPD and RV TDI-MPI (p = 0.01, R = 0.30) or septal TDI-MPI (p = 0.04, R = 0.24).
Bronchopulmonary dysplasia (BPD) is a chronic lung disorder that generally occurs in infants with prematurity treated with an artificial ventilation and oxygen therapy for acute respiratory failure. Severe BPD may result in chronic pulmonary artery hypertension (PAH) and gradual dilatation as well as hypertrophy of the right ventricle (RV)1)2)3)4)5) that can lead to RV dysfunction and right heart failure. It is associated with poor prognosis.3)4)5)6)7)8)
Cardiac catheterization is the gold standard for diagnosing PAH and grading its severity.9) However, it is an invasive procedure with significant complication rate, particularly in low body weight children. It is not suitable for evaluating PAH in infants with BPD.10)11) Therefore, echocardiographic assessments of PAH and RV dysfunction have been used in infants with BPD.9)12) By echocardiography, estimated systolic pulmonary artery pressure (sPAP) from tricuspid regurgitant jet velocity (TRJV) measurement is a useful parameter for evaluating PAH.3)4) However, one study has found a poor association between echocardiographic and cardiac catheterization measures of sPAP in infants with BPD.9) Although traditional echocardiographic methods such as RV ejection fraction and fractional change are widely used to evaluate RV dysfunction, these measurements are difficult due to the complex geometry of RV in small infants.3)
Pulse Doppler (PD)-derived myocardial performance index (MPI) has been proposed as a useful predictor of global myocardial performance13)14)15) as it measures combined systolic and diastolic function of the ventricles. It is a useful prognostic indicator in patients with congenital and acquired cardiac disease.16)17) Moreover, tissue Doppler (TD) imaging (TDI)-derived MPI simultaneously measures both contraction and relaxation velocities from the myocardium.18)19) that correlate with cardiac catheterization assessments of cardiac systolic and diastolic dysfunction of the RV. It is a prognosis predictive tool for RV dysfunction in patients with primary PAH.20)
One recent study in 21 infants with BPD has revealed that clinical severity of BPD is correlated with elevated RV E/E′ ratio (E: early tricuspid inflow velocity; E′: early tricuspid TD velocity) by TDI-MPI and abnormal left ventricular (LV) MPI.12) However, the main limitation of that study was that a relatively small sample size was used. In addition, they did not measure normal infants without BPD. Therefore, the objective of this study was to determine whether echocardiographic parameters such as TDI derived MPI measurements could provide sensitive measures for RV dysfunction that correlates with the severity of BPD.
We enrolled 45 patients diagnosed with BPD who were hospitalized at Chonnam National University Hospital from July 2010 to June 2012. Definition and grading of BPD was based on the Jobe Bancalari criteria as there was oxygen supplementation at 36 weeks of corrected gestational age in an infant who was at least 28 days old.1) We also enrolled 28 infants without BPD during the same period as controls. Patients with other primary diseases that could influence respiratory or cardiac function such as genetic disorders and congenital heart disease were excluded from this study. Patients with BPD were classified as mild, moderate, or severe BPD based on the NICHD/NHLBI/ORD workshop definition of BPD.1) BPD severity was classified according to the fraction of inspired oxygen (FiO2) or mechanical ventilation as follows: mild BPD (breath room air); moderate BPD (require oxygen supplementation FiO2 < 0.30); and severe BPD (FiO2 ≥ 0.30 or mechanical ventilation at 36 weeks of gestational age). Medical records and echocardiographic findings were reviewed.
Echocardiographic studies were performed at 35–37 weeks of corrected gestational age using M-mode, cross-sectional, color, and PD modalities with an 8–12 MHz transducer system (iE33 system, Philips Medical Systems, Best, the Netherlands).
The percent change in LV dimension of fractional shortening (FS) was calculated as: LV%FS = [(LVDd - LVDs) / LVDd] × 100, where LVDd was the LV dimension at end-diastole and LVDs was the LV dimension at end-systole. LV ejection fraction was calculated using the Teichholz method.21) Measurements of tricuspid velocity at the leaflet tips and TD tracings obtained on the RV free wall (RV TDI-MPIs) and the interventricular septum (septal TDI-MPIs) at the level of tricuspid valve annulus were performed from an apical four-chambered view (Fig. 1). RV MPI was estimated from tricuspid valve inflow and pulmonary valve outflow Doppler images. The time from cessation to the beginning of tricuspid inflow (a) and RV ejection time (b) were measured. RV MPI was calculated using the following equation: RV MPI = (isovolumic contraction time + isovolumic relaxation time) / ejection time = (a - b) / b.22) TDI-MPI was calculated from TD traces. Annular motion was recorded over at least three cardiac cycles at a sweep speed of 100 mm/sec.12)
Echocardiographic and clinical measures were compared between groups by one-way analysis of variance with the Kruskall-Wallis method and linear regression with a p value < 0.05 considered to be statistically significant. Data are expressed as mean ± standard deviation. Statistical analysis was performed using SPSS ver. 18.0 software (SPSS Inc., Chicago, IL, USA).
The demographic and clinical characteristic of patients used in this study are summarized in Table 1. Patients with BPD consisted of 21 boys and 24 girls with a mean gestational age of 27.7 ± 1.4 weeks. The mean corrected gestational age at the time of echocardiographic measurements was 36.6 ± 2.3 weeks. Among the 45 patients with BPD, 28 had mild BPD, 11 had moderate BPD, and 6 had severe BPD. The 28 patients without BPD (non-BPD group) consisted of 14 boys and 14 girls with mean gestational age of 29.4 ± 1.1 weeks. Their mean corrected gestational age at the time of echocardiographic measurements was 35.7 ± 2.2 weeks. Although gestational age and birth weight of the BPD group at birth were smaller than those of the non-BPD group, no significant difference in corrected gestational age or body weight was observed at the time of echocardiography. The mean number of mechanical ventilation days in the mild or moderate BPD group was higher than that in the non-BPD group (mild BPD: 24.8 ± 13.6 days; moderate BPD: 22.0 ± 17.8 days vs. non-BPD: 5.0 ± 4.6 days, p < 0.01, p = 0.01 vs. non-BPD, respectively). The mean number of oxygen treatment days in the mild, moderate, or severe BPD group was higher (p < 0.01) than that in the non-BPD group. The mean SpO2 at the time of echocardiographic evaluation were 94.8 ± 1.6% (moderate BPD) and 94.3 ± 1.9% (severe BPD) under supplement oxygen. The mean heart rate was not significantly (p > 0.05) different according to the severity of BPD. All patients had normal sinus rhythm. Diuretics and postnatal steroids were used in the moderate and severe BPD groups (Table 1). Among patients in the severe BPD group, 3 deaths (50%) were observed. The first patient received a tracheostomy at 60 days after birth because mechanical ventilator weaning failed. The patient died from sepsis at 132 days after birth. The second patient received sildenafil due to persistent and aggravated PAH although they received oxygen treatment 4 months after birth. The patient was admitted to intensive care unit (ICU) for mechanical ventilator care during the sildenafil and oxygen treatment due to aggravated dyspnea. The patient died on hospital day 8. The third patient was weaned from oxygen after improvement in BPD. However, this patient was admitted to the ICU at 4 months after birth due to acute respiratory difficulty caused by respiratory syncytial virus pneumonia. This patient received mechanical ventilator care but died on hospital day 7.
Results of LV function by standard M-mode echocardiography, tricuspid regurgitant (TR) pressure gradient, and PD-MPI in the three groups are shown in Table 2. The mean LV FS values in the non-BPD, mild BPD, moderate BPD, and severe BPD groups were 36.6 ± 6.1, 38.6 ± 5.8, 33.3 ± 6.8, and 40.8 ± 9.2%, respectively. The mean LV ejection fractions in the non-BPD, mild BPD, moderate BPD, and severe BPD groups were 69.5 ± 7.7, 72.1 ± 7.4, 64.9 ± 9.1, and 74.1 ± 11.7%, respectively. The mean TR pressure gradients in the non-BPD, mild BPD, moderate BPD, and severe BPD groups were 12.5 ± 8.9, 14.4 ± 7.6, 13.3 ± 11.6, and 8.8 ± 6.3 mm Hg, respectively. The mean PD-MPI values in the non-BPD, mild BPD, moderate BPD, and severe BPD groups were 0.26 ± 0.12, 0.28 ± 0.11, 0.36 ± 0.17, and 0.37 ± 0.21, respectively. No significant difference was found in LV FS, LV ejection fraction, TR pressure gradient, or PD-MPI between groups.
TD echocardiographic findings are summarized in Table 3. No significant difference in E/E′ ratio was observed between groups. The mean septal TDI-MPI of the severe BPD group (0.68 ± 0.06) was higher than that of the non-BPD group (0.58 ± 0.10, p < 0.01) or the mild BPD group (0.59 ± 0.12, p < 0.01). Moreover, the mean RV TDI-MPI of the severe BPD group (0.71 ± 0.13) was higher than that of the non-BPD group (0.56 ± 0.08, p < 0.05) or the mild BPD group (0.60 ± 0.12, p < 0.05) (Table 3, Fig. 2). However, mean septal TDI-MPI and RV TDI-MPI of the moderate BPD group were not significantly different from that of the non-BPD group or the mild BPD group.
Results of linear regression analysis demonstrated a significant correlation between BPD category and RV TDI-MPI (R = 0.30, p = 0.01, y = 0.04x + 0.55). The correlation between BPD category and septal TDI-MPI was also significant (R = 0.24, p = 0.04, y = 0.03x + 0.56) (Fig. 2).
No significant difference in mean LV ejection fraction (72.6 ± 16.2%), TR pressure gradient (8.0 ± 3.6 mm Hg), PD-MPI (0.28 ± 0.14), septal TDI-MPI (0.66 ± 0.02), or RV TDI-MPI (0.77 ± 0.13) was observed between patients (n = 3) who died and those with severe BPD who did not die.
In the current study, BPD severity was correlated with increased septal and RV TDI-MPI. The increase in RV TDI-MPI was found without a change in shortening fraction. Thus, our findings may be an earlier marker of cardiac dysfunction compared to traditional echocardiographic measurements that can change with the development of PAH.
PAH is a complication of premature neonates with BPD. Recent studies have increased the awareness that PAH can worsen the clinical course, morbidity, and mortality of BPD.3)4)5)23) Retrospective studies of infants with BPD-associated PAH have reported mortality rates of 14–38%.24)25) PAH is not just an advanced BPD marker. High pulmonary vascular resistance also can result in poor RV function with impaired cardiac output and increased pulmonary edema as a possible risk factor of sudden death. With strong correlations between PAH and survival of patients with BPD, early detection of PAH may provide useful prognostic parameter and allow earlier introduction of more appropriate respiratory support, cardiac medications, pulmonary arterial vasodilators, and surgical procedures to improve the outcomes.26) Echocardiography is non-invasive and widely available. It is currently the most commonly used screening modality for PAH in infants with BPD. TRJV is used to estimate sPAP. It represents the most common and reliable method to evaluate the presence and severity of PAH.3)4)5)23) Alternatives to measuring TRJV include assessing flattening of the interventricular septum, accelerated pulmonary regurgitation velocity, right atrial enlargement, or RV hypertrophy and dilation. One study demonstrated the utility of echocardiographic evaluation of PAH in infants with BPD.9) They showed that echocardiography can fail to detect measurable TRJV in a significant number of high-risk patients and that the absence of TRJV does not rule out the presence of severe PAH.9) We found no association between TRJV and BPD severity. In our study, 3 infants died in the severe BPD group. Two died during the follow-up period. Their deaths were attributed to PAH. However, echocardiographic parameters including PD and TDI-MPI performed at 35–37 weeks of corrected gestational age did not provide prognostic information for the risk of death.
RV MPI is the most powerful Doppler parameter that has distinguished 26 patients with primary pulmonary hypertension from 37 healthy individuals.27) One important limitation of this method is that it will limit the reliability in the presence of heart rate variation because the distance between the end and the onset of mitral inflow and ejection time was measured consecutively, not on the same heart cycle.28) In contrast, with the introduction of a modified index using TDI, we could measure the contraction and relaxation velocities simultaneously.18)
Various TD measurements are correlated with RV dysfunction as measured by cardiac catheterization in adults with pulmonary hypertension.29)30)31)32) Normative data for TD measurements have been published for children and fetuses. However, few data are available for children with pathology.33)34)35)36) One study in 21 infants with BPD has revealed that the clinical severity of BPD is correlated with an elevated RV E/E′ ratio by TDI-MPI. That study also revealed abnormal LV MPI. However, the RV and septal MPI were not correlated with BPD severity.12)
The limitations of our study include the relatively small sample size and its retrospective design. In addition, we could not obtain MPI parameters blindly to the severity of BPD. Moreover, TDI from the apical four-chamber view only accounts for cardiac movement within one dimension. It does not account for translational or circumferential movement. It could be affected by tracing angle position and heart rate. Furthermore, we did not measure tricuspid annular plane systolic excursion correlated with RV systolic function. We did not obtain data for LV diastolic function such as E, A, and E deceleration time through mitral inflow pulsed Doppler either. In addition, we did not check chorioamnionitis in our study, although it can contribute to the development of BPD. We did not check the development of pulmonary hypertension which can be associated with RV dysfunction after the diagnosis of BPD.
Nonetheless, our results revealed that an echocardiographic evaluation of RV function based on assessing the RV TDI-MPI could provide information on RV dysfunction in premature infants with BPD. Further longitudinal studies of TD echocardiographic assessments of cardiac function in premature infants with BPD are merited.
Figures and Tables
Fig. 1
The representative echocardiographic images of tissue Doppler imaging (TDI)-derived myocardial performance index (MPI) obtained from the interventricular septum (septal TDI-MPI) and the right ventricular (RV) free wall at the level of tricuspid annulus (RV TDI-MPI) in patients without bronchopulmonary dysplasia (BPD) or with mild, moderate, or severe BPD.

Fig. 2
The representative echocardiographic images of tissue Doppler imaging (TDI)-derived myocardial performance index (MPI) obtained from the interventricular septum (septal TDI-MPI, A) and the right ventricular (RV) free wall at the level of tricuspid annulus (RV TDI-MPI, B) in patients without bronchopulmonary dysplasia (BPD) or with mild, moderate, or severe BPD. Dots represent actual values. Lines represents linear regression.

Table 3
RV tissue Doppler imaging-derived myocardial performance index of prematurity without or with mild, moderate, or severe BPD
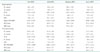
Data are mean ± standard deviation. *p < 0.05, †p < 0.01 vs. severe BPD. RV: right ventricular, BPD: bronchopulmonary dysplasia, E: early pulse Doppler wave velocity, E′: early tissue Doppler wave velocity, A′: late tissue Doppler wave velocity, S′: systolic tissue Doppler wave velocity, TDI: tissue Doppler imaging, MPI: myocardial performance index, ICT: isovolemic contraction time, IRT: isovolemic relaxation time
Acknowledgements
This study was supported by a grant (Industrial-educational Cooperation 2012) from the Korean Society of Echocardiography. This study was also supported by a grant (CRI 11083-31) from Chonnam National University Hospital Biomedical Research Institute and a grant (NRF-2015R1D1-A1A-01059017) of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Republic of Korea.
References
1. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163:1723–1729.
2. Keller RL, Ballard RA. Bronchopulmonary dysplasia. In : Gleason CA, Devaskar SU, editors. Avery's diseases of the newborn. 9th ed. Philadelphia: Elsevier Saunders;2012. p. 658–671.
3. Mourani PM, Mullen M, Abman SH. Pulmonary hypertension in bronchopulmonary dysplasia. Prog Pediatr Cardiol. 2009; 27:43–48.
4. Farquhar M, Fitzgerald DA. Pulmonary hypertension in chronic neonatal lung disease. Paediatr Respir Rev. 2010; 11:149–153.
5. Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. 2013; 37:124–131.
6. McLaughlin VV, Davis M, Cornwell W. Pulmonary arterial hypertension. Curr Probl Cardiol. 2011; 36:461–517.
7. Park JH, Park MM, Farha S, Sharp J, Lundgrin E, Comhair S, et al. Impaired global right ventricular longitudinal strain predicts long-term adverse outcomes in patients with pulmonary arterial hypertension. J Cardiovasc Ultrasound. 2015; 23:91–99.
8. Espinola-Zavaleta N, Soto ME, Romero-Gonzalez A, Gómez-Puente Ldel C, Muñoz-Castellanos L, Gopal AS, Keirns C, Lupi-Herrera E. Prevalence of congenital heart disease and pulmonary hypertension in Down's syndrome: an echocardiographic study. J Cardiovasc Ultrasound. 2015; 23:72–77.
9. Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008; 121:317–325.
10. Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004; 170:1006–1013.
11. Rosenzweig EB, Feinstein JA, Humpl T, Ivy DD. Pulmonary arterial hypertension in children: diagnostic work-up and challenges. Prog Pediatr Cardiol. 2009; 27:4–11.
12. Yates AR, Welty SE, Gest AL, Cua CL. Myocardial tissue Doppler changes in patients with bronchopulmonary dysplasia. J Pediatr. 2008; 152:766–770. 770.e1
13. Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol. 1995; 26:135–136.
14. Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol. 1995; 26:357–366.
15. Eidem BW, Tei C, O'Leary PW, Cetta F, Seward JB. Nongeometric quantitative assessment of right and left ventricular function: myocardial performance index in normal children and patients with Ebstein anomaly. J Am Soc Echocardiogr. 1998; 11:849–856.
16. Eto G, Ishii M, Tei C, Tsutsumi T, Akagi T, Kato H. Assessment of global left ventricular function in normal children and in children with dilated cardiomyopathy. J Am Soc Echocardiogr. 1999; 12:1058–1064.
17. Tsutsumi T, Ishii M, Eto G, Hota M, Kato H. Serial evaluation for myocardial performance in fetuses and neonates using a new Doppler index. Pediatr Int. 1999; 41:722–727.
18. Harada K, Tamura M, Toyono M, Oyama K, Takada G. Assessment of global left ventricular function by tissue Doppler imaging. Am J Cardiol. 2001; 88:927–932. A9
19. Ouss AJ, Riezebos RK. The tissue Doppler imaging derived post-systolic velocity notch originates at the aortic annulus. J Cardiovasc Ultrasound. 2014; 22:23–27.
20. Grignola JC, Ginés F, Guzzo D. Comparison of the Tei index with invasive measurements of right ventricular function. Int J Cardiol. 2006; 113:25–33.
21. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976; 37:7–11.
22. Ko JH, Eom GH, Cho HJ, Nam KI, Ma JS, Kook H, Cho YK. Right ventricular myocardial performance index is decreased with severe pressure-overload cardiac hypertrophy in young rats. Pediatr Cardiol. 2013; 34:1556–1566.
23. Bland RD. Neonatal chronic lung disease in the post-surfactant era. Biol Neonate. 2005; 88:181–191.
24. Kim DH, Kim HS, Choi CW, Kim EK, Kim BI, Choi JH. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012; 101:40–46.
25. An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, Kim HS, Choi JH, Noh CI, Yun YS. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010; 40:131–136.
26. Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007; 120:1260–1269.
27. Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward SB. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996; 9:838–847.
28. Yasuoka K, Harada K, Toyono M, Tamura M, Yamamoto F. Tei index determined by tissue Doppler imaging in patients with pulmonary regurgitation after repair of tetralogy of Fallot. Pediatr Cardiol. 2004; 25:131–136.
29. Moustapha A, Lim M, Saikia S, Kaushik V, Kang SH, Barasch E. Interrogation of the tricuspid annulus by Doppler tissue imaging in patients with chronic pulmonary hypertension: implications for the assessment of right-ventricular systolic and diastolic function. Cardiology. 2001; 95:101–104.
30. Boissiere J, Gautier M, Machet MC, Hanton G, Bonnet P, Eder V. Doppler tissue imaging in assessment of pulmonary hypertension-induced right ventricle dysfunction. Am J Physiol Heart Circ Physiol. 2005; 289:H2450–H2455.
31. Emilsson K, Loiske K. Isovolumetric relaxation time of the right ventricle assessed by tissue Doppler imaging. Scand Cardiovasc J. 2004; 38:278–282.
32. Hsiao SH, Lee CY, Chang SM, Yang SH, Lin SK, Huang WC. Pulmonary embolism and right heart function: insights from myocardial Doppler tissue imaging. J Am Soc Echocardiogr. 2006; 19:822–828.
33. Frommelt PC, Ballweg JA, Whitstone BN, Frommelt MA. Usefulness of Doppler tissue imaging analysis of tricuspid annular motion for determination of right ventricular function in normal infants and children. Am J Cardiol. 2002; 89:610–613.
34. Mori K, Nakagawa R, Nii M, Edagawa T, Takehara Y, Inoue M, Kuroda Y. Pulsed wave Doppler tissue echocardiography assessment of the long axis function of the right and left ventricles during the early neonatal period. Heart. 2004; 90:175–180.
35. Rychik J, Tian ZY. Quantitative assessment of myocardial tissue velocities in normal children with Doppler tissue imaging. Am J Cardiol. 1996; 77:1254–1257.
36. Iwashima S, Sekii K, Ishikawa T, Itou H. Serial change in myocardial tissue Doppler imaging from fetus to neonate. Early Hum Dev. 2013; 89:687–692.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download