Abstract
A 58-year-old man had been diagnosed with non-obstructive hypertrophic cardiomyopathy (HCMP) according to echocardiography findings 16 years ago. Echocardiography showed ischemic cardiomyopathy (CMP)-like features with decreased systolic function but a non-dilated chamber. Coronary angiography was performed but showed a normal coronary artery. Cardiac magnetic resonance imaging (MRI) revealed multifocal transmural and subepicardial delayed-enhancing areas at the anteroseptal, septal, and inferoseptal left ventricular (LV) wall, and wall thinning and decreased motion of the anteroseptal LV wall. Findings of ischemic CMP-like features by echocardiography suggested microvascular dysfunction. This late stage of HCMP carries a high risk of sudden death. Cardiac MRI evaluation may be necessary in cases of ischemic CMP-like features in HCMP. In this case, the diagnosis of end-stage HCMP with microvascular dysfunction was confirmed by using cardiac MRI after a follow-up period of more than 16 years.
The clinical expression of hypertrophic cardiomyopathy (HCMP) has proved to be particularly heterogeneous with a diverse disease spectrum.1)2) The end stage of HCMP, characterized by a left ventricular (LV) ejection fraction (EF) < 50% at rest, reflects global systolic dysfunction.3) However, there has been limited information reported on the progression of HCMP to myocardial ischemia and remodeling.
Here, we report a patient with HCMP who progressed to ischemic cardiomyopathy (CMP)-like features as suggested by echocardiography findings after a follow-up period of more than 16 years. We diagnosed this case as the end stage of HCMP with microvascular dysfunction by using cardiac magnetic resonance imaging (MRI).
The patient was a 42-year-old man when he was first hospitalized as a result of atypical chest discomfort and electrocardiographic abnormality (ST depression in II, III, and aVF). He received an echocardiography examination, and he was diagnosed with septal hypertrophied and non-obstructive HCMP (EF, 72%; septal thickness, 19.7 mm; posterior wall LV thickness, 10 mm; LV end-diastolic/systolic volume, 42.7/13.4 mL). Coronary angiography (CAG) showed a normal coronary artery. His mother had been diagnosed with apical hypertrophy, but there was no history of sudden death in any family member. He was discharged with beta-blocker.
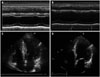
The patient was hospitalized after 7 years because of syncope. Holter monitoring findings were infrequent ventricular premature contraction. A neurologic examination indicated normal results. Echocardiographic findings were non-obstructive HCMP with decreased LV wall thickness (16 mm) and low-normal EF values (EF = 63.9%). He was discharged with beta-blocker and angiotensin receptor blocker. The patient was rehospitalized 9 years later because of atypical chest discomfort. His blood pressure was 121/80 mm Hg, pulse rate was 77/min, and respiratory rate was 20/min. Echocardiography revealed akinesis at the basal to mid anteroseptal, basal to mid septal, basal to mid inferior, and basal to mid anterior LV wall motion with decreased LV systolic function (EF = 45.5%) but normal chamber size (LV dimension diastole/systole, 54.8/42.3 mm) (Fig. 1). A treadmill test revealed positive findings. Electrocardiographic changes showed horizontal ST depression > 2 mm on II, III, and aVF, and ST elevation on aVR and V1 during exercise. During the recovery phase, electrocardiography showed down-sloping ST depression and T wave inversions on II, III, and aVF.
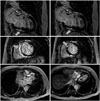
To evaluate the underlying cause of the regional wall motion abnormality, CAG was performed but showed a normal coronary artery. Cardiac MRI revealed multifocal transmural and subepicardial delayed enhancing areas at the anteroseptal, septal and inferoseptal LV wall with wall thinning and decreased motion of the anteroseptal LV wall (Fig. 2). Echocardiographic findings from several examinations during the follow-up period are shown in Table 1. He was diagnosed with end-stage HCMP with microvascular dysfunction.
HCMP is widely regarded as a disease predominantly associated with hyperdynamic LV systolic function in the absence of another cardiac or systemic disease (e.g., hypertension or aortic stenosis) capable of producing the magnitude of hypertrophy evident.4) HCMP is not rare and is the most common genetic cardiovascular disease.1) Remodeling of the LV chamber in HCMP has been shown to occur in several clinical circumstances, including progression of LV hypertrophy during adolescence. 5)
A small distinctive subset of HCMP patients (approximately 5–10%) show the evolution into the end stage (or "burned-out" phase), characterized by LV wall thinning, cavity enlargement and systolic dysfunction that resembles dilated CMP and produces a relentlessly progressive and irreversible heart failure.1)6) In 1998, Chang et al.7) demonstrated two cases of HCMP progressing to dilated CMP-like features after a follow-up period of more than 15 years with echocardiographic evidence, but there was no cardiac MRI evaluation. Chang et al.7) concluded that pathologic study of more cases is necessary to reveal the pathogenesis of the functional and morphological changes of the myocardium. In the previous study, end stage of HCMP among 44 cases was identified approximately after 14 ± 18 years.3)
This case that we encountered in 1999, diagnosed on echocardiography and CAG, had hypertrophied LV walls but a non-dilated LV chamber. After a follow-up period of more than 16 years, echocardiographic findings from several examination were akinesis at the basal to mid anteroseptal, basal to mid septal, and basal to mid inferior, and basal to mid anterior LV wall motion with decreased LV systolic function (EF = 45%) but a normal chamber size. A treadmill test showed positive results. We considered the possibility of HCMP progressing to ischemic CMP-like features after a follow-up period of more than 16 years with echocardiographic evidence. However, the coronary artery was normal on CAG. Cardiac MRI revealed multifocal transmural and subepicardial delayed enhancing areas. Therefore, we diagnosed end stage of HCMP with fibrosis. However, it is unclear what progressed the end stage of HCMP by the cause of the pathologic mechanism. We consider that microvascular dysfunction is a common feature of HCMP and reflects the interplay of a variety of mechanisms, including reduced arteriolar density, fibrosis, and myocyte disarray.8) Microvascular dysfunction promotes blunted myocardial blood flow, leading to recurrent myocardial ischemia, replacement fibrosis, and possibly adverse LV remodeling.9) In patients with HCMP, the degree of microvascular dysfunction is a strong, independent predictor of clinical deterioration and death.10)
Similar to that observed in this patient, end-stage HCMP is an unfavorable complication with a mortality rate of 11% per year and a risk of sudden death.3) Therefore, patients with marked disease progression and LV remodeling eventually become candidates for primary prevention of sudden death with implantable defibrillators and evaluation for heart transplantation. 2) Cardiac MRI evaluation may be necessary to guide these important treatment decisions. The reason cardiac MRI is essential is that it provides contrast visualization of late gadolinium enhancement (LGE), generally considered indicative of myocardial fibrosis.11) Maron and Spirito6) said that LGE, presumably representing the consequences of longstanding microvascular ischemia, and resulting in myocyte death and ultimately replacement fibrosis as a repair process, to be evident predominantly in thinner segment of LV and associated with abnormalities of wall motion.12) There is also an inverse relationship between systolic function and LGE on cardiac MRI.2)12) In patients with HCMP, a subset of patients with low-normal EF values (50–65%) are identified on contrast-enhanced cardiac MRI as having substantial degrees of LGE, suggesting a transition phase, potentially heralding the beginning of advanced LV remodeling and systolic dysfunction.
In conclusion, the present case demonstrated non-obstructive HCMP that progressed to ischemic CMP-like features diagnosed on echocardiography and cardiac MRI after a follow-up period of more than 16 years. This case was confirmed as the end stage of HCMP with fibrosis on cardiac MRI. Therefore, we may consider the end stage of HCMP in case of systolic and/or diastolic dysfunction with a variety of wall motion abnormality on echocardiography but normal coronary artery.
Figures and Tables
Fig. 1
After 7 years, transthoracic echocardiography M-mode image (A), four chamber view (C), after 16 years, M-mode image (B), and four chamber view (D).
References
1. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002; 287:1308–1320.
2. Olivotto I, Maron BJ, Appelbaum E, Harrigan CJ, Salton C, Gibson CM, Udelson JE, O'Donnell C, Lesser JR, Manning WJ, Maron MS. Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. 2010; 106:261–267.
3. Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006; 114:216–225.
4. Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J Am Coll Cardiol. 1995; 26:1699–1708.
5. Biagini E, Coccolo F, Ferlito M, Perugini E, Rocchi G, Bacchi-Reggiani L, Lofiego C, Boriani G, Prandstraller D, Picchio FM, Branzi A, Rapezzi C. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J Am Coll Cardiol. 2005; 46:1543–1550.
6. Maron BJ, Spirito P. Implications of left ventricular remodeling in hypertrophic cardiomyopathy. Am J Cardiol. 1998; 81:1339–1344.
7. Chang KH, Ha JW, Chung NS, Cho SY. Progression of hypertrophic cardiomyopathy to dilated cardiomyopathy--a case reports and review of the literatures. Yonsei Med J. 1998; 39:61–66.
8. Lorenzoni R, Gistri R, Cecchi F, Olivotto I, Chiriatti G, Elliott P, McKenna WJ, Camici PG. Coronary vasodilator reserve is impaired in patients with hypertrophic cardiomyopathy and left ventricular dysfunction. Am Heart J. 1998; 136:972–981.
9. Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004; 43:2260–2264.
10. Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003; 349:1027–1035.
11. Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003; 41:1561–1567.
12. Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, De Santis M, Quarta G, Nistri S, Cecchi F, Salton CJ, Udelson JE, Manning WJ, Maron BJ. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008; 52:559–566.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download