Abstract
Cardiac magnetic resonance imaging (CMR) is a useful diagnostic imaging modality in patients with known or suspected coronary artery disease (CAD). It provides unique information not available from other modalities, however, it is complex. CMR is not a single technique. Instead, it consists of multiple distinct techniques and a lack of understanding of which techniques to perform and how to interpret the findings in combination limits its efficacy and widespread use. Conversely, its multiparametric nature can provide a comprehensive assessment with the potential for higher accuracy than is achievable by other modalities. Moreover, its ability to directly assess myopathic processes often contributes insights that change patient management. In this article we provide a brief technical overview and focus on specific clinical scenarios in patients with known or suspected CAD. We highlight the multiparametric nature of CMR and discuss cases which illustrate the unique information that CMR can contribute.
Cardiac magnetic resonance imaging (CMR) is a useful diagnostic imaging modality in patients with known or sus-pected coronary artery disease (CAD). Numerous studies have shown its efficacy in identifying the presence of CAD and its sequelae, and for risk stratifying patients for future outcome.1)2)3) In many conditions, such as following acute myocardial infarction (MI), CMR often provides unique information not available from other modalities.1) Nonetheless, CMR has limited availability and is considered by some to be a 'boutique' modality. One possible reason is its complexity. CMR is not a single technique. Instead, it consists of multiple distinct techniques and a lack of understanding of which techniques to perform and how to interpret the findings in combination limits the efficacy of CMR. On the other hand, its multiparametric nature can provide a comprehensive assessment with the potential for higher accuracy than is achievable by other modalities. In this context, this article presents a brief technical overview of CMR along with a discussion of how CMR can be used in patients with known or suspected CAD. Rather than providing a detailed summary of the literature, we will emphasize a 'real-world' perspective in a working clinical practice. We will focus on specific scenarios which highlight the unique information that CMR provides and discuss illustrative cases.
The multiplicity of techniques and the variety of information that can be obtained in a CMR exam can be exhaustive, but increasingly there has been a move towards standardization of imaging protocols tailored to specific indications, an effort spearheaded by the Society of Cardiovascular Magnetic Resonance.4) Our suggested implementation and a timeline (Fig. 1) of a CMR protocol for a standard exam is as follows:
Cine images provide comprehensive evaluation of regional and global ventricular function and overall cardiac morphology. Visual evaluation of valvular function and morphology is also performed using these images. Cine images are typically acquired in the short-axis plane from above the mitral valve through the left ventricular (LV) apex along with standard 2-, 3-, and 4-chamber long-axis views. Inter- and intra-observer reproducibility of cine CMR imaging for the quantification of LV volumes and function have been shown in multiple studies to be excellent, predominantly due to its high spatial and temporal resolution and its capacity for complete LV coverage. The improvement in reproducibility relative to echocardiography allows a significant reduction in the sample sizes required for research studies to demonstrate meaningful changes as a result of experimental therapies. This has led to increasing use of CMR in research studies that use cardiac morphology and/or function as an efficacy endpoint.5)
These sequences are designed to demonstrate contrast media passage through the myocardium in a manner that reliably reflects myocardial blood flow. The sequences are heavily T1-weighted, in order to accurately depict the passage of a T1 shortening contrast agent such as gadolinium through the myocardium. It is most often used for the detection of obstructive CAD, where it is performed with pharmacological vasodilation (e.g., adenosine or regadenoson). The underlying principle is similar to that in nuclear perfusion imaging, where a vasodilator is used to accentuate regional differences in myocardial blood flow. However, as opposed to nuclear techniques, CMR perfusion imaging is a first pass imaging study that directly images the passage of contrast, and therefore is performed using an abbreviated adenosine protocol (~3 minutes). Also, CMR perfusion imaging has higher spatial resolution (> 20 ×) than radionuclide techniques, and can depict a perfusion defect that is limited to the subendocardial layer. CMR perfusion imaging has the additional advantage of providing a more linear depiction of myocardial blood flow in response to vasodilation, without the plateau phenomenon seen with nuclear agents.6)
Delayed enhancement (DE) imaging allows the diagnosis and sizing of MI, assessment of viability, and other tissue characterization such as identification of thrombus and nonischemic scarring. Images are obtained 5–15 minutes after the ad-ministration of gadolinium contrast, and views are matched to the spatial location of the cine images. The DE technique accentuates the visibility of areas with abnormal gadolinium accumulation by using an inversion pulse to "null" the signal from normal myocardium, resulting in abnormal areas standing out as regions of bright, "hyperenhanced" signal intensity against a background of very dark normal myocardium. The standard high-resolution DE sequence is acquired from a segmented acquisition (data from multiple heart beats), but in cases of ar-rhythmia or inability to breath hold, single-shot techniques can provide comparable data in a fraction of the imaging time with a small reduction in sensitivity.7)
The technique of DE-CMR has been extensively validated. In animal models, DE-CMR has been shown to demonstrate acute and chronic MI with a near exact spatial match to histopathology specimens.8) Additionally, these studies show that DE-CMR can distinguish between reversible and irreversible injury independent of wall motion, infarct age, and reperfusion status. Compared with single photon emission computed tomography (SPECT) imaging, the DE-CMR technique is significantly more sensitive for the detection of subendocardial infarction, over 40% of which are missed with SPECT.9) With standard imaging parameters, DE-CMR is capable of demonstrating infarcts involving as little as one one-thousandth of total LV mass, and that are undetectable by techniques that assess myocardial perfusion or contractile function. The high spatial resolution of DE-CMR has been used to visualize microinfarctions, involving as little as 1 g of tissue, which may occur during otherwise successful percutaneous coronary intervention.10)
An overview of cardiac and great vessel anatomy is helpful in many cases and can be rapidly performed using single-shot dark blood and/or bright blood techniques. These result in a stack of still-frame images and are usually acquired in the standard orthogonal imaging planes (axial, sagittal, or coronal).
T1 and T2 mapping techniques are increasingly being investigated in studies of infarction as well as other myocardial disorders, and can be performed before and/or after the administration of intravenous contrast. These sequences provide a quantitative assessment of regional myocardial T1 and T2 values, and are less subject to surface coil sensitivity profiles that can result in variable image intensity for different regions of the heart. In the absence of gadolinium contrast, T1 and T2 values are increased in the setting of acute necrosis-related edema, and thus these sequences allow the depiction of edema and provide a metric, which may be useful for purposes of quantification and serial assessment. A parametric map of extracellular volume fraction can be made by combining pre-contrast ("native") and post-contrast T1 mapping values.11) Extracellular volume fraction will be increased both in the setting of acute necrosis and chronic collagenous scar. These sequences are also being investigated for use in a variety of non-ischemic myocardial disorders, such as amyloidosis and Anderson-Fabry disease.12)13)
Each of the sequences described above provide separate and distinct pieces of information. Hence, one can get multiple data acquisitions of the same location and obtain a comprehensive, multifaceted view of the heart. In general, if two (or more) tests provide information regarding the presence of a disease, diagnostic accuracy is not necessarily improved compared with one test alone. For example, if the algorithm used to determine the presence of disease requires both tests to be positive, this improves diagnostic specificity at the cost of sen-sitivity. Conversely, if the algorithm requires only one test to be positive, this generally improves sensitivity but worsens specificity. With multiparametric CMR acquisitions, it is important to realize that each of the sequences described above are 'tuned' to highlight specific biological tissues or properties (e.g., infarction, thrombus, fat, tissue perfusion, etc.). Accordingly, depending on the property, the results of one test should take precedence over the results of another, and algorithms can be developed to improve diagnostic accuracy, which is not true ordinarily regarding multiple tests. Additionally, because many image artifacts are pulse-sequence specific, these are not propagated throughout the examination and generally do not reduce overall scan quality.
The report by Klem et al.14) demonstrates an example of an interpretation algorithm that uses multiparametric acquisitions to improve diagnostic accuracy. The algorithm is based on 2 principles. First, with perfusion-CMR and DE-CMR, we have independent methods to obtain information regarding the presence or absence of MI. Thus, one method could be used to confirm the results of the other. Second, DE-CMR image quality (e.g., signal-to-noise ratio), is far better than perfusion-CMR since it is less demanding in terms of scanner hardware (DE-CMR images can be built up over several seconds rather than in 0.1 seconds as is required for first-pass perfusion). Thus, DE-CMR is far more accurate for the diagnosis of MI. Conceptually, it then follows that perfusion defects that have similar intensity and extent during both stress and rest ("matched defect") but do not have infarction on DE-CMR are artifactual and should not be considered positive for CAD. Conversely, the presence of infarction on DE-CMR favors the diagnosis of CAD even if the results of perfusion imaging are equivocal. The algorithm and some typical images are dis-played in Fig. 2. Overall, the combination of perfusion and DE images (compared with perfusion images alone) improved the accuracy for the diagnosis of CAD from 68% to 88% in a cohort of patients with intermediate pretest probability of obstructive CAD.
Because of late presentation, patients with MI may present without diagnostic biomarker elevation of electrocardiogram abnormalities. Moreover, wall motion abnormalities on cardiac imaging may not occur unless the infarcted region exceeds 20% to 50% of the myocardial wall thickness. Similarly, scintigraphic defects may not be apparent until greater than 10 g of tissue is infarcted. Thus, because a sizable threshold of damage is required, echocardiography or SPECT may miss MI, particularly when it is small or subendocardial. Wall motion abnormalities may also occur in entities other than MI, such as Takotsubo cardiomyopathy, indicating a lack of specificity. In these instances, where the diagnosis of MI is difficult, DE-CMR may prove helpful. It is notable that DE-CMR is the only imaging modality for the detection of MI that has been validated in a multicenter trial. In an international study of 282 patients with acute and 284 with chronic first-time MI, the sensitivity of DE-CMR for the detection of MI reached 99% and 94% in acute and chronic MI, respectively.15)
One might postulate that the MIs not recognized by standard criteria are likely small, and of uncertain significance. However, in the report by Kwong et al.,16) the presence of unrecognized MI detected by DE-CMR was associated with a greater than 6-fold higher risk of major adverse cardiac events compared to the absence of such an MI. Importantly, the information from DE-CMR was a stronger predictor of outcome than standard clinical risk factors and even catheterization data. Similarly, the study by Kim et al.2) reported that the presence of unrecognized MI by DE-CMR predicted an 11-fold higher risk of all-cause mortality than those without MI.
Fig. 3 demonstrates CMR images in a patient with palpitations who did not have any symptoms or signs of CAD. A stress echocardiogram was normal, but a Holter examination demonstrated frequent episodes of non-sustained ventricular tachyarrhythmia, which were asymptomatic. The patient was thought to have a structurally normal heart and the arrhythmia was thought to be right ventricular outflow tract in origin. The CMR images showed normal LV size and systolic function, however, there was a focal subendocardial infarct in the mid-ventricular anterior wall, which was completely unexpected. A subsequent cardiac catheterization demonstrated occlusive coronary disease in the second diagonal branch of left anterior descending artery.
The diagnostic performance of stress perfusion CMR has been evaluated in a number of studies in humans. A recent meta-analysis reveals that on average, the sensitivity and specificity of perfusion-CMR for detecting obstructive CAD were 89% and 76%, respectively.17) Additionally, several studies have directly compared the performance of adenosine stress perfusion CMR with SPECT imaging. In the largest single-center trial to date, the Clinical evaluation of MAgnetic Resonance imaging in Coronary heart disease (CE-MARC) trial, 752 patients underwent SPECT and CMR, as well as invasive coronary angiography. The study found that the sensitivity of CMR for the detection of obstructive CAD exceeded that of SPECT (87% vs. 67%), with preserved specificity (both 83%).18) It is important to recognize that the CMR examinations in this study were multi-parametric and involved several components including cine and DE imaging in addition to stress and rest perfusion imaging. Similar to the study by Klem et al.,14) the finding of an infarct on DE images alone was sufficient to render the CMR test positive for CAD. It is likely that a key factor in the high performance of CMR was the multi-parametric information that was provided. Combined with the superior delineation of infarction provided by DE-CMR relative to SPECT, it is not surprising that many centers are now using stress perfusion CMR as a first-line test.
Coronary magnetic resonance angiography (MRA) may be used to directly visualize coronary anatomy and morphology. However, coronary magnetic resonance imaging is technically demanding, leading to intermediate sensitivity and specificity values for the detection of CAD in validation studies. More recently, "whole-heart" free-breathing steady-state free precession techniques in combination with multichannel coils and parallel acquisition has improved coronary MRA.19) The higher inherent signal-to-noise that is available from 3T scanners is likely to improve coronary MRA further.20) That said, imaging times are still relatively long (7–10 minutes) and image quality can be highly variable between patients. The CE-MARC trial also included a coronary MRA component. Of the 676 patients that had coronary MRA performed, it is notable that only 55% of coronary artery segments (15 segments for each patient) were deemed sufficient quality to analyze. Overall, the diagnostic accuracy of CMR did not change if the coronary MRA component was excluded. Hence, we believe that reports that suggest that coronary MRA has similar diagnostic accuracy with cardiac computed tomography angiography are premature. From a "working" clinical practice perspective, currently we do not perform coronary MRA as part of our routine CMR examination except in patients with suspected coronary anomalies.
Similar to troponin, the detection of injury by DE-CMR is specific for irreversible myocardial damage but is not specific for MI. One potential advantage of DE-CMR is that the pattern of hyperenhancement, rather than simply the presence or extent, may offer important information regarding the etiology of myocardial damage. For this purpose, the concept that ischemic myonecrosis proceeds as a wavefront from the subendocardium to the epicardium with increasing coronary occlusion time is crucial. Correspondingly, hyperenhancement patterns that spare the subendocardium and are limited to the middle or epicardial portion of the LV wall are usually nonischemic in origin because significant damage in the setting of CAD almost always involves the subendocardium. Moreover, certain nonischemic disorders, such as myocarditis, amyloidosis, hypertrophic cardiomyopathy, have characteristic hyperenhancement patterns that suggest specific diagnoses, and a systematic approach to interpreting DE-CMR images in patients with cardiomyopathy has been proposed.21)
However, one caveat should be mentioned. Novices should be cautioned against overzealously interpreting non-CAD-type hyperenhancement. If there is a large area of hyperenhancement and it is entirely midwall or epicardial throughout its course, then this is clearly a non-CAD-type pattern. Likewise, if there are numerous islands of midwall or epicardial hyperenhancement throughout the LV and crossing into multiple coronary artery territories, this is also likely nonischemic (Fig. 4). However, a single isolated 'dot' of hyperenhancement or a few patchy lesions of hyperenhancement in a limited focal region of myocardium (Fig. 4) is unlikely to be nonischemic in origin, regardless of whether it spares the subendocardium or not. In our experience, the latter pattern is more likely to represent an aborted MI or atherosclerotic (or embolic) disease affecting a small secondary coronary branch, rather than a nonischemic disorder.
DE-CMR has proven to be an excellent technique for the evaluation of patients with heart failure and/or suspected to have a cardiomyopathy. In this setting it may alleviate the need for invasive coronary angiography. However, the use of hyperenhancement patterns should not be considered merely another way to rule-in or rule-out CAD. By allowing a direct assessment of myopathic processes, tissue characterization with DE-CMR often provides a clue as to the specific etiology. Even when definitive clinical tests have already been performed, CMR can contribute information that is unique and often clinically relevant.
Fig. 5 shows representative images in 3 patients presenting with chest discomfort and ST-segment elevation. In all 3, troponins were elevated, hence the diagnosis was ST-segment elevation MI. However, invasive coronary angiography demonstrated normal coronary arteries. CMR was performed because the diagnosis was uncertain, and in each case tissue character-ization with DE-CMR contributed unique insights to the clinical situation. In these 3 cases, CAD had already been ruled-out by coronary angiography, however, without the information provided by CMR to clarify the diagnosis, patient management and potentially prognosis would have been crucially changed.
Finally, a more in-depth case is illustrated in Fig. 6. This is of a 57-year-old man who underwent CMR before a scheduled radiofrequency ablation procedure for paroxysmal atrial fibrillation. The primary purpose of the CMR was to delineate the pulmonary vein anatomy, however, at our institution, a cardiac study is routinely performed in addition to evaluate for structural heart disease. The patient complained of palpitations, but otherwise was asymptomatic, and the physical examination was normal. His cardiac history was significant for known single-vessel coronary disease, which had been diagnosed by invasive coronary angiography one year prior during an admission for chest pain. Otherwise, he was healthy and he did not have any significant past medical history. An echocardiogram performed 2 days prior to the CMR demonstrated normal LV size and function, normal valvular function, and only mild LV wall hypertrophy (Fig. 6A). Cine-CMR demonstrated similar findings to the echocardiogram (not shown). Remarkably, delayed-enhancement images demonstrated widespread hyperenhancement from the base of the LV to nearly the apex. The hyperenhancement was primarily epicardial (right ventricular side of septum), however there was also subendocardial hyperenhancement of the basal LV lateral wall, which was less severe (Fig. 6B). The DE-CMR findings were consistent with a diffuse infiltrative process such as amyloidosis or sarcoidosis, hence a cardiac biopsy was performed. The biopsy demonstrated transthyretin amyloidosis. Recall that this patient presented with a history of paroxysmal atrial fibrillation and single-vessel CAD and had no other known medical problems. Now he has a biopsy-proven diagnosis of cardiac amyloidosis, which raises the question: without CMR, how long would it have taken to get the diagnosis?
For patients with known or suspected CAD, the conventional use of noninvasive imaging is to rule-in or rule-out obstructive CAD or to assess the extent and severity of myocardial ischemia. While CMR can be an accurate alternative to other modalities in this regard, there are many patients that do not fit this conventional scenario. We have discussed a variety of cases where invasive coronary angiography had already been performed, yet the diagnosis remained unclear. CMR is often utilized in these situations, where it is considered the final arbitrator for the diagnosis. Although well intentioned, this can pigeonhole CMR as an important but secondary test only to be used when there are discrepancies among conventional tests. This is shortsighted. To paraphrase Donald Rumsfeld, the former United States Secretary of Defense, the primary problem is "you don't know, what you don't know". In our experience when CMR is used as a first-line test, unexpected information similar to but perhaps less dramatic than that described in the last case, occurs nearly on a weekly basis. In the majority of these patients, CMR does not decide between two possible diagnoses as much as it provides an entirely new diagnosis. In countries such as the United States, where cost to the patient for a CMR examination is no more expensive than that of SPECT,22) we believe that CMR should considered a first-line examination for the evaluation of patients with known or suspected CAD.
Figures and Tables
Fig. 1
Timeline and potential components of a multitechnique CMR examination including stress and rest perfusion imaging. Note that the entire exam can be performed within 30–45 minutes. In cases where the patient is unable to cooperate, the cine examination can be performed with real-time imaging and delayed enhancement with single-shot techniques. This technique will reduce artifacts in patients unable to breath-hold and total exam time will be shortened further. Adapted from Kim et al. J Am Coll Cardiol 2009;55:1-16, with permission of Elsevier.1) CMR: cardiac magnetic resonance imaging, MI: myocardial infarction, MRA: magnetic resonance angiography, SNR: signal to noise ratio, ECV: extra-cellular volume fraction.

Fig. 2
Interpretation algorithm for incorporating delayed enhancement-cardiac magnetic resonance imaging (DE-CMR) with stress and rest perfusion magnetic resonance imaging (MRI) for the detection of coronary disease. A: Schema of the interpretation algorithm. 1) Positive DE-CMR study: hyperenhanced myocardium consistent with a prior myocardial infarction (MI) is detected. Does not include isolated midwall or epicardial hyperenhancement which can occur in nonischemic disorders. 2) Standard negative stress study: no perfusion defects at stress or rest. 3) Standard positive stress study: perfusion defects are present with adenosine that are absent or reduced at rest. 4) Artifactual perfusion defect: matched stress and rest perfusion defects without evidence of prior MI on DE-CMR. B: Patient examples. Top row: patient with a positive DE-CMR study demonstrating an infarct in the inferolateral wall (red arrows) although perfusion-MRI is negative. The interpretation algorithm classifies this patient as positive for coronary artery disease (CAD). Coronary angiography verified disease in a circumflex marginal artery. Middle row: patient with a negative DE-CMR study but with a prominent reversible defect in the anteroseptal wall on perfusion-MRI (red arrows). The interpretation algorithm classifies this patient as positive for CAD. Coronary angiography demonstrated a proximal 95% LAD stenosis. Bottom row: patient with a matched stress-rest perfusion defect (blue arrows) but without evidence of prior MI on DE-CMR. The interpretation algorithm classifies the perfusion defects as artifactual. Coronary angiography demonstrated normal coronary arteries. Adapted from Klem et al. J Am Coll Cardiol 2006;47:1630-8, with permission of Elsevier.14) LCX: left circumflex artery, LAD: left anterior descending.
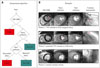
Fig. 3
Cine and delayed-enhancement images in a patient without signs or symptoms of coronary artery disease who was believed to have a structurally normal heart. Images demonstrate a focal subendocardial infarct (arrows) with normal left ventricular size and systolic function. CMR: cardiac magnetic resonance imaging, DE: delayed enhancement.

Fig. 4
The cartoon schematic on the left demonstrates multiple islands of hyperenhancement in a diffuse, near global pattern, which is classic for particular types of viral myocarditis. In other words, the correct interpretation of this pattern would be "non-CAD-type". In contrast, it would be incorrect to judge the pattern on the right, which shows only a few "dots" of hyperenhancement in a limited, focal territory, as non-CAD-type. CAD: coronary artery disease, DE: delayed enhancement.
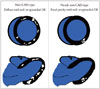
Fig. 5
Typical delayed enhancement-cardiac magnetic resonance (DE-CMR) images from 3 patients with chest discomfort, ST-segment elevation, positive troponins, and normal invasive coronary angiograms. A: Linear, epicardial hyperenhancement (yellow arrows) is present and is indicative of myocarditis. B: Cine and DE images of a patient with sudden emotional stress and apical ballooning; the absence of hyper-enhancement is consistent with Takotsubo cardiomyopathy. C: Focal but transmural hyperenhancement (red arrow) involving the lateral apex is present and indicative of myocardial infarction (MI) because of temporary occlusion of a small diagonal branch off the distal left anterior descending coronary artery. DE-CMR with a long inversion time (600 ms) shows a thrombus (yellow arrowhead) in the left atrial appendage, suggesting that an embolus led to the MI.
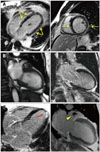
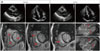
Fig. 6
A 57-year-old patient with paroxysmal atrial fibrillation undergoing cardiac magnetic resonance imaging (CMR) prior to radiofrequency ablation procedure. A: Echocardiogram demonstrated normal left ventricular (LV) size and function and only mild LV wall hypertrophy. B: Delayed enhancement-CMR images demonstrated widespread hyperenhancement (arrows) primarily in a non-coronary artery disease-type pattern, which suggested an infiltrative process.
References
1. Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2009; 55:1–16.
2. Kim HW, Klem I, Shah DJ, Wu E, Meyers SN, Parker MA, Crowley AL, Bonow RO, Judd RM, Kim RJ. Unrecognized non-Q-wave myocardial infarction: prevalence and prognostic significance in patients with suspected coronary disease. PLoS Med. 2009; 6:e1000057.
3. Shah R, Heydari B, Coelho-Filho O, Murthy VL, Abbasi S, Feng JH, Pencina M, Neilan TG, Meadows JL, Francis S, Blankstein R, Steigner M, di Carli M, Jerosch-Herold M, Kwong RY. Stress cardiac magnetic resonance imaging provides effective cardiac risk reclassification in patients with known or suspected stable coronary artery disease. Circulation. 2013; 128:605–614.
4. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013; 15:91.
5. Grothues F, Braun-Dullaeus R. Serial assessment of ventricular morphology and function. Heart Fail Clin. 2009; 5:301–314. v
6. Gerber BL, Raman SV, Nayak K, Epstein FH, Ferreira P, Axel L, Kraitchman DL. Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. J Cardiovasc Magn Reson. 2008; 10:18.
7. Sievers B, Elliott MD, Hurwitz LM, Albert TS, Klem I, Rehwald WG, Parker MA, Judd RM, Kim RJ. Rapid detection of myocardial infarction by subsecond, free-breathing delayed contrast-enhancement cardiovascular magnetic resonance. Circulation. 2007; 115:236–244.
8. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999; 100:1992–2002.
9. Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003; 361:374–379.
10. Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001; 103:2780–2783.
11. White SK, Sado DM, Flett AS, Moon JC. Characterising the myocardial interstitial space: the clinical relevance of non-invasive imaging. Heart. 2012; 98:773–779.
12. Banypersad SM, Sado DM, Flett AS, Gibbs SD, Pinney JH, Maestrini V, Cox AT, Fontana M, Whelan CJ, Wechalekar AD, Hawkins PN, Moon JC. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2013; 6:34–39.
13. Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G, Fontana M, Maestrini V, Flett AS, Robson MD, Lachmann RH, Murphy E, Mehta A, Hughes D, Neubauer S, Elliott PM, Moon JC. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging. 2013; 6:392–398.
14. Klem I, Heitner JF, Shah DJ, Sketch MH Jr, Behar V, Weinsaft J, Cawley P, Parker M, Elliott M, Judd RM, Kim RJ. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006; 47:1630–1638.
15. Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, Parker M, Napoli A, Judd RM. Gadoversetamide Myocardial Infarction Imaging Investigators. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation. 2008; 117:629–637.
16. Kwong RY, Schussheim AE, Rekhraj S, Aletras AH, Geller N, Davis J, Christian TF, Balaban RS, Arai AE. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003; 107:531–537.
17. Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, Nelemans PJ, Schalla S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012; 59:1719–1728.
18. Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012; 379:453–460.
19. Kato S, Kitagawa K, Ishida N, Ishida M, Nagata M, Ichikawa Y, Katahira K, Matsumoto Y, Seo K, Ochiai R, Kobayashi Y, Sakuma H. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J Am Coll Cardiol. 2010; 56:983–991.
20. Yang Q, Li K, Liu X, Du X, Bi X, Huang F, Jerecic R, Liu Z, An J, Xu D, Zheng H, Fan Z, Li D. 3.0T whole-heart coronary magnetic resonance angiography performed with 32-channel cardiac coils: a single-center experience. Circ Cardiovasc Imaging. 2012; 5:573–579.
21. Senthilkumar A, Majmudar MD, Shenoy C, Kim HW, Kim RJ. Identifying the etiology: a systematic approach using delayed-enhancement cardiovascular magnetic resonance. Heart Fail Clin. 2009; 5:349–367. vi
22. Shah DJ, Kim HW, Kim RJ. Evaluation of ischemic heart disease. Heart Fail Clin. 2009; 5:315–332. v




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download