Abstract
Hypertrophic obstructive cardiomyopathy (HOCM) patients with severe left ventricular outflow tract (LVOT) obstruction (those with a gradient of > 100 mm Hg) are at the highest risk of hemodynamic deterioration during pregnancy. Complications of HOCM include sudden cardiac death, heart failure, and arrhythmias. Physiological changes during pregnancy may induce these complications, affecting maternal and fetal health conditions. Therefore, close monitoring with appropriate management is essential for the well-being of both mother and fetus. We report on the case of a 27-year-old female patient with severe LVOT obstruction HOCM, pressure gradient (PG) of 125 mm Hg at resting, and 152 mm Hg induced by the Valsalva maneuver at 34 weeks gestation. This case showed how close monitoring using echocardiography and proper management during the course of pregnancy resulted in successful delivery in the patient with extremely high PG HOCM.
Hypertrophic cardiomyopathy (HCM) is a genetic cardiovascular disease characterized by primary hypertrophy of a non-dilated ventricle. Hypertrophic obstructive cardiomyopathy (HOCM) is generally regarded as a left ventricular (LV) outflow tract (LVOT) pressure gradient (PG) of approximately 50 mm Hg at rest or with provocative maneuvers.1) Complications of HOCM include sudden cardiac death, heart failure, and arrhythmia.2)
In HOCM patients, the cardiovascular system is exposed to acute hemodynamic burdens during pregnancy, which may lead to unfavorable complications.3) Therefore, special monitoring and management is required for HOCM patients during pregnancy.
We report on a case of a 27-year-old female patient of HOCM with high LVOT PG over 100 mm Hg, as known as severe LVOT obstruction,4) who maintained pregnancy and successful delivery through close monitoring using transthoracic echocardiography (TTE).
A 27-year-old primigravid female was referred at 9 weeks gestation for further management of known HCM. One year ago, she was admitted to another hospital for chest tightness. She was diagnosed with HCM, but no further treatment was administered at that time. She had no previous medical history, and no family history of any cardiovascular disorder or sudden cardiac death. Initially she felt palpitations rarely, but after pregnancy, she complained of exertional dyspnea and palpitation upon fast walking or overeating as 9 weeks gestation.
Physical examination showed blood pressure (BP) of 93/64 mm Hg and regular pulse rate of 108 beats per minute. Breathing was stable. She had a grade III/VI systolic harsh murmur at the third intercostal space on the left sternal border (Erb's area). Electrocardiography showed sinus rhythm. The baseline TTE was performed at 11 weeks gestation (Fig. 1).
TTE showed asymmetrical hypertrophy (maximal thickness at septum 15 mm) with dynamic LVOT obstruction due to systolic anterior motion of the mitral valve, and a severely enlarged left atrium (volume index; 57 mL/m2). Hyperdynamic LV systolic function (ejection fraction 78%), high LVOT PG (peak/mean PG; 75/47 mm Hg at rest, 103/52 mm Hg during Valsalva maneuver), eccentric moderate mitral regurgitation (MR) grade III/IV, and pseudonormalization of LV filling pattern (E/e'; 24) were also observed. In summary, TTE showed HOCM with high LVOT PG and MR grade III/IV.
Initially, twenty-four-hour Holter monitoring showed basically normal sinus rhythm with rare atrial premature complexes (APC) and ventricular premature complexes (VPC).
After reaching 15 weeks of pregnancy, she complained of exertional dyspnea upon fast walking and dizziness on orthostatic position change, such as standing up quickly from a seated position. Due to low BP, we decided to follow her progress without pharmacologic therapy.
Repeat TTE performed at 17 weeks of gestation showed an increase in PG of 119/52 mm Hg at resting and 147/70 mm Hg on the Valsalva maneuver. She experienced dizziness and palpitations, which occasionally seemed to last for an entire day. Twenty-four-hour Holter monitoring was performed repeatedly. Average heart rate was 91 beats per minute, faster than before, frequent APC's (5568/130599, 4.26%) up to non-sustained atrial tachycardia and occasional VPC's (522, 0.40%) up to triplets were checked. Due to an increase of LVOT PG on TTE and detection of frequent arrhythmias, pharmacologic therapy was considered. However, the patient's BP was still low. We recommended lifestyle modification including avoiding dehydration or excessive effort, and referral from a private clinic to our obstetrics department for a multidisciplinary approach.
As fetal ultrasound findings were normal, obstetricians recommended pharmacologic therapy. Bisoprolol 1.25 mg twice a day was prescribed at 22 weeks of pregnancy, and the chest discomfort was improved. At 34 weeks gestation, TTE showed LVOT PG of 125/63 mm Hg at resting and 152/72 mm Hg on the Valsalva maneuver (Fig. 2). Considering her symptoms and need for fetal growth, delivery was planned at 37 weeks of gestation. TTE performed just before delivery showed high LVOT PG of 115/66 mm Hg at resting and PG of 152/83 mm Hg on the Valsalva maneuver. She continued taking bisoprolol until she proceeded to delivery and avoiding use of sympathomimetics or inotropics was recommended. Two days later, caesarean section with general anesthesia was performed to give a birth to a healthy baby without cardiovascular complication. Just after delivery, TTE showed a decrease in LVOT PG of 87/33 mm Hg at rest and she was unable to perform the Valsalva maneuver (Fig. 3).
Thirteen months after delivery, a follow-up TTE still showed high LVOT PG of 100/31 mm Hg at resting and 169/45 mm Hg on the Valsalva maneuver. She had developed progressive symptoms of dyspnea on exertion and chest pain. Because of drug-refractory symptoms and severe LVOT obstruction, we decided to perform an alcohol septal ablation for improving quality of life. The procedure was done successfully and she discharged without significant complications (Fig. 4).
HCM is the most common genetic cardiovascular disorder, with prevalence of approximately 0.2% (i.e., 1:500) in the general population.5) This case showed how to monitor and manage a pregnancy with severe LVOT obstruction (PG over 100 mm Hg).
Pregnancy causes physiologic changes in the cardiovascular system which included increases in blood volume (up to 40%) and cardiac output (30–50%), and reductions in systemic vascular resistance and BP.6) In the first and second trimesters, the increase of cardiac output is influenced by a larger stroke volume. But later in pregnancy, a faster heart rate affects increase of cardiac output. Enlarged ventricular cavity which is induced by increasing volume reduce the LVOT obstruction theoretically, however, cardiac output countervail against this effect, the LVOT gradient will increase with advancing gestation.6)7)
HCM is considered a World Health Organization (WHO) class II–III in modified WHO classification of maternal cardiovascular risk, implying that depending on individual condition, there is a low to high risk of complications.8) Cardiovascular complication rate in pregnancy with HCM appears to increase with history of previous cardiac events, poor functional class (New York Heart Association III or IV), severe LV systolic dysfunction, and LVOT obstruction.9)
The higher the LVOT gradient is before pregnancy or during the first trimester, the higher the likelihood that symptoms will progress. In addition, the subset of patients with severe LVOT obstruction (those with a gradient of 100 mm Hg) are at the highest risk of hemodynamic deterioration during pregnancy.4)
Pharmacologic management is recommended in symptomatic patients with HCM such as angina or dyspnea.10) Beta blockers are mainly recommended as first-line agents because of their negative inotropic effects and decreasing adrenergic-induced tachycardia. Oral diuretics may be added with congestion symptoms.10) Our patient showed low BP during pregnancy and a small amount of amniotic fluid was observed on fetal ultrasound at 26 weeks of gestation. Accordingly, only low dose beta blocker was administered and a modified lifestyle, avoiding aggravating factors such as excessive activity and dehydration was recommended.
In our case, the patient had extremely high LVOT PG more than 100 mm Hg, resulting in a high risk of maternal cardiovascular complications. Because of her symptoms, close follow-up monitoring was performed using TTE every two weeks during her third trimester. And we planned a caesarean section with consideration of maternal symptoms, fetal growth, and LVOT PG on TTE. As a result, regular TTE monitoring was performed and severe cardiovascular complications and disease progression were prevented.
In conclusion, we report on a case of a 27-year-old pregnant female with HOCM who presented with extremely high LVOT PG. She was monitored closely with regular follow-up of TTE during pregnancy to determine the timing of caesarean section, which eventually resulted in a successful delivery.
Complications such as sudden cardiac death, heart failure, and arrhythmia may occur in HOCM patients either throughout the course of pregnancy or during labor and delivery. TTE is a relatively safe method which does not cause harm to either the mother or the fetus. Therefore, close monitoring using TTE and management with medical treatment seems essential in pregnant patients with HOCM before and during pregnancy.
Figures and Tables
Fig. 1
Baseline transthoracic echocardiography (TTE). TTE showed massive asymmetric septal hypertrophy of left ventricular septum with wall thickness > 22 mm in parasternal long axis view (A), eccentric mitral regurgitation grade III/IV (B), systolic anterior motion (white arrow) of mitral valve presented by M-mode (C), very high left ventricular outflow tract pressure gradient at resting (D), and during the Valsalva maneuver (E).
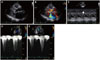
Fig. 2
Serial check up of left ventricular outflow tract (LVOT) pressure gradient (PG) presented by M-mode at resting (A) and during the Valsalva maneuver (B). At 11 weeks gestation, initial transthoracic echocardiography examination showed LVOT PG (peak/mean PG; 75/47 mm Hg at rest, 103/52 mm Hg during Valsalva maneuver). As the pregnancy progresses, when 34 weeks gestation, LVOT PG was increasing up to 125/63 mm Hg at resting and 152/72 mm Hg on the Valsalva maneuver.
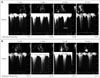
Fig. 3
Serial follow up of left ventricular outflow tract (LVOT) pressure during pregnancy. This graph shows the serial values of LVOT PG at resting (blue line) and during the Valsalva maneuver (red line) which changes according to the gestational age. PG: pressure gradient.

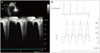
Fig. 4
Transthoracic echocardiography (TTE) and cardiac catheterization just before the alcohol septal ablation. TTE showed severe left ventricular outflow tract (LVOT) pressure gradient (PG) (A) and cardiac catheterization showed also high LVOT PG (127 mm Hg) at the same time (B). Ao: aorta, LV: left ventricle.
References
1. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002; 287:1308–1320.
2. Tanaka H, Kamiya C, Katsuragi S, Tanaka K, Miyoshi T, Tsuritani M, Yoshida M, Iwanaga N, Neki R, Yoshimatsu J, Ikeda T. Cardiovascular events in pregnancy with hypertrophic cardiomyopathy. Circ J. 2014; 78:2501–2506.
3. Shim WJ. Role of echocardiography in the management of cardiac disease in women. J Cardiovasc Ultrasound. 2014; 22:173–179.
4. Alegria JR, Nishimura R. Hypertrophic cardiomyopathy and pregnancy. In : Oakley C, Warnes CA, editors. Heart disease in pregnancy. Oxford: Blackwell Publishing;2007. p. 173–185.
5. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995; 92:785–789.
6. Thaman R, Varnava A, Hamid MS, Firoozi S, Sachdev B, Condon M, Gimeno JR, Murphy R, Elliott PM, McKenna WJ. Pregnancy related complications in women with hypertrophic cardiomyopathy. Heart. 2003; 89:752–756.
7. Pieper PG, Walker F. Pregnancy in women with hypertrophic cardiomyopathy. Neth Heart J. 2013; 21:14–18.
8. European Society of Gynecology (ESG). Association for European Paediatric Cardiology (AEPC). German Society for Gender Medicine (DGesGM). Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L. ESC Committee for Practice Guidelines. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32:3147–3197.
9. Krul SP, van der Smagt JJ, van den Berg MP, Sollie KM, Pieper PG, van Spaendonck-Zwarts KY. Systematic review of pregnancy in women with inherited cardiomyopathies. Eur J Heart Fail. 2011; 13:584–594.
10. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011; 58:e212–e260.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download