Abstract
Barth syndrome (BTHS) is a rare genetic disorder characterized by various types of cardiomyopathy, neutropenia, failure to thrive, skeletal myopathy, and 3-methylglutaconic aciduria. BTHS is caused by loss-of-function mutations in the tafazzin (TAZ) gene located on chromosome Xq28, leading to cardiolipin deficiency. We report a 13-month-old boy with BTHS who had a novel de novo mutation in the TAZ gene. To the best of our knowledge, this is the first reported case of a BTHS patient with a de novo mutation in Korea. This report will contribute towards expanding the knowledge on the mutation spectrum of the TAZ gene in BTHS.
Barth syndrome [BTHS (MIM 302060)], first described as a mitochondrial disease affecting neutrophils as well as cardiac and skeletal muscles, is a rare X-linked recessive disorder characterized by cardiomyopathy (CMP), neutropenia, failure to thrive, skeletal myopathy and 3-methylglutaconic aciduria.1)2) BTHS is caused by loss-of-function mutations of the tafazzin (TAZ) gene located on chromosome Xq28. In the present report, we describe a Korean patient with BTHS, who had a novel de novo mutation in the TAZ gene. This is the first reported case of a BTHS patient with a de novo mutation in Korea.
A 13-month-old boy was referred to our hospital with intractable heart failure with dilated CMP, to evaluate the necessity of cardiac transplantation. He was born at full term with a birth weight of 3.0 kg. He had no siblings and had no family history of CMP or sudden death. At the age of 6 months, he had been admitted to another hospital because of lethargy, tachypnea with respiratory distress, and mild fever. Rhinovirus was isolated from a nasopharyngeal swab by real-time polymerase chain reaction. Chest radiography had shown marked cardiomegaly with pulmonary edema and echocardiogram had revealed a dilated left ventricle (LV) (LV end-diastolic diameter of 38.6 mm) with 30% ejection fraction (EF) and moderate mitral regurgitation (MR). Under the impression of dilated CMP due to acute myocarditis, the patient had been prescribed digoxin, furosemide, spironolactone and captopril and discharged from the hospital after stabilization of his hemodynamic status.
At presentation to our hospital at 13 months of age, the patient's body weight and height were 7.8 kg (< 3rd percentiles) and 72.5 cm (< 3rd percentiles), respectively. The characteristic facial features of BTHS were not observed. He could walk unaided and say "mama" and "papa". On physical examination, grade II/VI regurgitant systolic murmur was heard at the apex. Laboratory investigations revealed a white blood cell count of 2740/µL with an absolute neutrophil count of 438/µL. Serum concentrations of troponin I and lactate were 1.597 ng/mL (reference range: 0–0.3 ng/mL) and 3.8 mmol/L (reference range: 0.7–2.5 mmol/L), respectively. Serum electrolyte, sugar, liver function tests, creatine kinase (CK), and CK-MB levels were normal. The B-type natriuretic peptide level was 765 pg/mL. Urine organic acid analysis showed elevated levels of 3-methylglutaconic acid at 58.4 mmol/mol creatinine (reference range: 0–19 mmol/mol creatinine) and 3-methyglutaric acid at 7.5 mmol/mol creatinine (reference range: not detected). Chest X-ray revealed marked cardiomegaly with pulmonary edema (Fig. 1), and electrocardiogram showed normal sinus rhythm with left ventricular hypertrophy (Fig. 2). Echocardiography showed a dilated and hypertrophied spherical LV (LV end-diastolic diameter of 37.7 mm; z score: +4.6) with 23% EF, moderate MR, prominent endomyocardial trabeculations, LV non-compacted/compacted end-diastolic ratio of 2.3, color Doppler flow within the deep intertrabecular recesses and no prominent papillary muscle, confirming the diagnosis of dilated CMP with LV non-compaction (Fig. 3). At the age of 18 months, carvedilol was added, starting at 0.05 mg/kg/dose twice a day, and was gradually increased to 0.2 mg/kg/dose twice a day. No significant side effects of the medications were noted. After 1 year later from adding carvedilol, LV systolic function was remarkably improved (Fig. 3 and 4). Echocardiography showed a dilated LV with an EF of 53%, LV end-diastolic diameter of 35.6 mm (z score: +3.1), and mild MR (Fig. 4). During 2 years of follow-up, the patient had to be hospitalized twice for influenza infection and unspecified upper respiratory infection. Mild neutropenia persisted throughout the follow-up period (absolute neutrophil count of 150–650/µL). His characteristic clinical manifestations suggested BTHS. Gene sequence analysis revealed a hemizygous in-frame deletion of 9 amino acids within exon 10 of the TAZ gene, c.725_751del (p.Pro242_Glu250del). The mutation was not present in the Human TAZ Gene Mutation and Variation Database,3) and the variant was not detected in the patient's mother, indicating that it was a de novo mutation (Fig. 5). The patient was stable at his last outpatient visit at the age of 29 months. His body weight and height were 9.8 kg (< 3rd percentiles) and 81.5 cm (< 3rd percentiles), respectively. He could run well as well as ascend and descend stairs, but was still unable to speak beyond "mama" and "papa".
BTHS is a rare X-linked recessive disorder with clinical features of various types of CMP, neutropenia, skeletal myopathy, and 3-methylglutaconic aciduria. It has an estimated prevalence of 1 in 300000–400000 live births.1)3)4) The primary genetic defect in BTHS is mutations in the TAZ, which consists of 11 exons and is located at Xq28.5)6) This mutation lead to cardiolipin (CL) deficiency.2)7) The TAZ gene encodes TAZ with sequence homology to acyltransferases involved in phospholipid metabolism.8) TAZ is a mitochondrial protein located in the inner mitochondrial membrane and plays a crucial role in CL remodeling.2)8)9) CL is a predominant phospholipid of the mitochondrial inner membrane; it plays an important role in maintaining mitochondrial structure and is also involved in cellular apoptosis.7)10) Thus far, more than 200 different mutations in TAZ gene have been identified.2)3) Only 15% of boys carry de novo mutations that are not identified in their mother's somatic DNA.3) The correlations between the genotype and the phenotype have not yet been studied. There is no known racial or ethnic predilection for this mutation.6) However, the fact that the majority of BTHS patients have been reported from Europe, North America, and Australia suggests that the disease may be under-diagnosed.11)12) In Korea, one patient with BTHS was recently identified; he died after contrast dye injection for computed tomographic angiography.13) Our patient had a novel de novo mutation that was a hemizygous in-frame deletion in his TAZ gene, c.725_751del (p.Pro242_Glu250del), which was not inherited from his mother. To the best of our knowledge, this is the first reported case of a BTHS patient with a de novo mutation in Korea. The genetic analysis of this case was conducted by the help of the National Supporting Program for Genetic Diagnosis of Rare Diseases of the Korea Centers for Disease Control and Prevention. Infections due to neutropenia and cardiac complications are likely to be the major causes of morbidity and mortality in patients with BTHS.14) The survival rates of BTHS patients are gradually increasing mainly because of improved control of its complications such as heart failure and neutropenia.15) Early diagnosis of this condition is very important, since previous reports have shown that the mortality rate in patients who are identified prospectively and managed proactively decreases from 70% (retrospectively diagnosed patients) to just 10%.16) Despite this fact, a mean lag time between the first presentation and diagnosis of BTHS was 3.3 years.6) Our patient, too, was diagnosed after 12 months from the onset of symptom. He presented to us with acute circulatory collapse and respiratory viral infection. The patient was initially misdiagnosed with myocarditis/dilated CMP. The initial presentation of BTHS-associated CMP may mimic viral myocarditis/CMP or be precipitated by viral infection.6) His management was focused on controlling heart failure, and he showed a good response to medications including beta-blockers. Data from the literature suggest that heart failure can often be prevented by standard medical therapy in patients with BTHS, and the patients may remain stable for several years or may even improve over time like our patient, although most patients need to be maintained on standard cardiac medications for CMP.14)17) Our patient showed neutropenia at initial presentation, but this finding was mistakenly considered as the side effect of medications or bone marrow suppression due to frequent viral infections, because we thought that the initial diagnosis of acute myocarditis with proven etiology was definite. Therefore, BTHS should be included in the differential diagnosis of males presenting with CMP of apparent viral etiology, especially when neutropenia is present.6) Our patient's clinical course was unique in that he showed no gross motor delay over 2 years of age. He could walk unaided at the age of 12 months, unlike most patients with BTHS who achieve this milestone by the age of 2 years, and could run well at his last follow-up. Skeletal myopathy is widely recognized in BTHS, and most boys have at least mildly delayed gross motor milestones.6)14)
In conclusion, we identified a novel de novo mutation in the TAZ gene, c.725_751del (p.Pro242_Glu250del), which is the first genetically confirmed case of BTHS in a Korean male.
Figures and Tables
Fig. 1
Chest X-ray shows marked cardiomegaly with pulmonary edema at admission (A) and decreased cardiomegaly at the last follow-up (B).
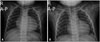
Fig. 3
Echocardiographic images in apical four chamber (A) and parasternal short axis view (B) show a marked dilated and globular left ventricle, prominent endomyocardial trabeculations, and color Doppler flow within the deep intertrabecular recesses (C).
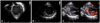
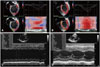
Fig. 4
Peak systolic longitudinal strain by speckle tracking in apical four chamber view and M-mode in standard parasternal long axis view. First (A and C) and last (B and D) echocardiographic image of the index patient. During follow up period, the cardiac systolic function remarkably improved over time.
References
1. Barth PG, Scholte HR, Berden JA, Van der Klei-Van Moorsel JM, Luyt-Houwen IE, Van't Veer-Korthof ET, Van der Harten JJ, Sobotka-Plojhar MA. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci. 1983; 62:327–355.
2. Aprikyan AA, Khuchua Z. Advances in the understanding of Barth syndrome. Br J Haematol. 2013; 161:330–338.
3. barthsyndrome.org [Internet]. Human TAZ gene variants database. updated 2015 Mar 28. cited 2015 Jul 10. Available from: http://www.barthsyndrome.org./science--medicine/human-taz-gene-variants-database.
4. Kelley RI, Cheatham JP, Clark BJ, Nigro MA, Powell BR, Sherwood GW, Sladky JT, Swisher WP. X-linked dilated cardiomyopathy with neutropenia, growth retardation, and 3-methylglutaconic aciduria. J Pediatr. 1991; 119:738–747.
5. Bolhuis PA, Hensels GW, Hulsebos TJ, Baas F, Barth PG. Mapping of the locus for X-linked cardioskeletal myopathy with neutropenia and abnormal mitochondria (Barth syndrome) to Xq28. Am J Hum Genet. 1991; 48:481–485.
6. Clarke SL, Bowron A, Gonzalez IL, Groves SJ, Newbury-Ecob R, Clayton N, Martin RP, Tsai-Goodman B, Garratt V, Ashworth M, Bowen VM, McCurdy KR, Damin MK, Spencer CT, Toth MJ, Kelley RI, Steward CG. Barth syndrome. Orphanet J Rare Dis. 2013; 8:23.
7. Barth PG, Valianpour F, Bowen VM, Lam J, Duran M, Vaz FM, Wanders RJ. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am J Med Genet A. 2004; 126A:349–354.
8. Neuwald AF. Barth syndrome may be due to an acyltransferase deficiency. Curr Biol. 1997; 7:R465–R466.
9. Vreken P, Valianpour F, Nijtmans LG, Grivell LA, Plecko B, Wanders RJ, Barth PG. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem Biophys Res Commun. 2000; 279:378–382.
10. Jefferies JL. Barth syndrome. Am J Med Genet C Semin Med Genet. 2013; 163C:198–205.
11. Sakamoto O, Kitoh T, Ohura T, Ohya N, Iinuma K. Novel missense mutation (R94S) in the TAZ (G4.5) gene in a Japanese patient with Barth syndrome. J Hum Genet. 2002; 47:229–231.
12. Cantlay AM, Shokrollahi K, Allen JT, Lunt PW, Newbury-Ecob RA, Steward CG. Genetic analysis of the G4.5 gene in families with suspected Barth syndrome. J Pediatr. 1999; 135:311–315.
13. Kim GB, Kwon BS, Bae EJ, Noh CI, Seong MW, Park SS. A novel mutation of the TAZ gene in Barth syndrome: acute exacerbation after contrast-dye injection. J Korean Med Sci. 2013; 28:784–787.
14. Spencer CT, Bryant RM, Day J, Gonzalez IL, Colan SD, Thompson WR, Berthy J, Redfearn SP, Byrne BJ. Cardiac and clinical phenotype in Barth syndrome. Pediatrics. 2006; 118:e337–e346.
15. Rigaud C, Lebre AS, Touraine R, Beaupain B, Ottolenghi C, Chabli A, Ansquer H, Ozsahin H, Di Filippo S, De Lonlay P, Borm B, Rivier F, Vaillant MC, Mathieu-Dramard M, Goldenberg A, Viot G, Charron P, Rio M, Bonnet D, Donadieu J. Natural history of Barth syndrome: a national cohort study of 22 patients. Orphanet J Rare Dis. 2013; 8:70.
16. Huhta JC, Pomerance HH, Barness EG. Clinicopathologic conference: Barth syndrome. Fetal Pediatr Pathol. 2005; 24:239–254.
17. Mazurová S, Tesařová M, Magner M, Houšt'ková H, Hansíková H, Augustínová J, Tomek V, Vondráčková A, Zeman J, Honzík T. Novel mutations in the TAZ gene in patients with Barth syndrome. Prague Med Rep. 2013; 114:139–153.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download