Abstract
Stress-induced cardiomyopathy has become a more recognized and reported entity. It can be caused by emotional or physical stress, which causes excessive catecholamine release. Typically, the clinical course is benign with conservative treatment being effective. However, stress-induced cardiomyopathy can be fatal. A 41-year-old female presented with cardiogenic shock followed by sudden back pain. Initial echocardiographic finding showed severely decreased ejection fraction with akinesia at all mid-to-apical walls with relatively preserved basal wall contractility. The coronary artery was intact on coronary angiography. Cardiac resuscitation and extra-corporeal membrane oxygenation was needed to manage the cardiogenic shock. Recovery was complete after 2 weeks.
Stress-induced cardiomyopathy (SCMP; also termed takotsubo cardiomyopathy, broken heart syndrome, or apical ballooning syndrome) accounts for 1.7–2.2% of cases of suspected acute coronary syndrome. SCMP has become increasingly recognized and reported.1)2) It can be precipitated or caused by wide range of emotional stresses, like death of relatives or a pet, public speaking, fierce arguments, or natural disasters,3) or by physical stresses including subarachnoid hemorrhage, traumatic brain injury, and critical illness, which cause excessive catecholamine release, although a triggering event is not always present.4) Usually, SCMP has a benign course with mortality as low as 1–3%, and most cases feature complete resolution of left ventricular (LV) function over several weeks. In those cases, only conservative treatment is sufficient.3) But, SCMP can be fatal, and recent studies show that SCMP on certain conditions had an increased rate of severe acute complications.5) Hypotension and shock develop due to severe systolic dysfunction with or without LV outflow tract obstruction which could influence the choice of treatment.
We experienced an extreme case of SCMP that presented as cardiogenic shock in a young woman. No risk factors were evident. Eventually, after extra-corporeal membrane oxygenation (ECMO), recovery was complete.
A 41-year-old female visited the emergency room with severe back pain without specific cause, which began the day before. She had no past medical history, did not drink alcohol and had never smoked.
Physical examination revealed mild periumbilical tenderness without rebound tenderness. There was no tenderness or limitation of range of motion in the back. Initial vital signs were non-specific, with mild tachypnea (systolic blood pressure/diastolic blood pressure 117/86 mm Hg, pulse rate 86 beats per min, respiratory rate 24 breaths per min, and body temperature 36.0℃).
Serial laboratory work-up was done to find serologic abnormalities. No significant abnormalities were apparent on blood test. Complete blood count (CBC) showed monocytosis [white blood cell (WBC) 7200/mm3, neutrophils 65.9%, monocytes 13.6%], and slightly elevated hemoglobin level (15.2 g/dL). Platelet count was normal (199000/mm3). Other findings including liver enzymes were within normal limits. No active lung lesion was detected on chest X-ray.
A computed tomography scan of the abdomen and pelvis with contrast revealed an adhesion of uterus fundus to the anterior abdominal wall with uncomplicated renal cyst and mild bronchiectasis in both lower lobes. But we could not find a definite lesion that could arouse pain. The patient received conservative treatment including pain control and was discharged.
The following morning, the patient revisited the emergency room with aggravated back pain with new-onset cyanosis on both arms and legs. Follow-up laboratory work-up including cardiac enzymes revealed increased creatine kinase MB (53.43 ng/mL), troponin-T (0.285 ng/mL), and pro-brain natriuretic peptide (2023 pg/mL). Creatine phosphokinase and lactate dehydrogenase was elevated (1827 IU/L and 363 IU/L, respectively). Follow-up CBC count showed leukocytosis (WBC 15900/mm3, neutrophils 75.4%, monocytes 7.5%). The hemoglobin level was unchanged (15.2 g/dL) and platelet count was normal (230000/mm3). Electrolyte levels indicated hyponatremia (sodium 131 mmol/L, potassium 4.5 mmol/L, chloride 100 mmol/L). Thyroid function test was within normal limits (thyroid stimulating hormone 0.664 IU/mL, free thyroxine 1.38 ng/dL). Electrocardiographic findings revealed sinus tachycardia (heart rate 110 beats per min) with ST segement elevation in precordial leads (Fig. 1). Viral markers including hepatitis B, C and influenza A and B antigen were all negative. Transthoracic echocardiography (TTE) revealed akinesia at each apex, all mid-LV walls and hypokinesia at all basal LV walls with severely decreased LV ejection fraction and intact right ventricular function. LV cavity size was normal, and there wasn't pericardial effusion. There also were mild mitral regurgitation, minimal aortic regurgitation, tricuspid regurgitation on color Doppler. And there were no regional edema and ventricular wall thickening suggesting myocarditis (Fig. 2). Overall, echocardiographic findings were compatible with SCMP or possible ischemic heart disease (Supplementary movie 1 and 2). Metabolic acidosis was apparent on arterial blood gas analysis (pH 6.885, HCO4 6.9 mmol/L) with hypotension (systolic blood pressure/diastolic blood pressure 80/50 mm Hg, heart rate 134 beats per min, respiratory rate 20 per min, body temperature 36.0℃). After resuscitation using fluid and an inotropic agent, coronary angiography was performed. No significant coronary arterial stenosis was apparent (Fig. 3). During the coronary angiography, the patient's condition abruptly deteriorated. Cardiac resuscitation was administered for 11 min. Recovery of spontaneous circulation was not achieved, so ECMO was begun via the right femoral artery and vein. After inserting right femoro-femoral ECMO catheter, right foot showed a cyanotic color change, prompting a switch to central venous artery ECMO with LV venting via a lower sternal J-sternotomy approach on the same day.
On the third day of admission, despite full ECMO support, progressive metabolic acidosis with hypotension and high central venous pressure (23 mm Hg) with generalized edema developed, indicating ECMO malfunction. The same day, the sternal wound reopened. Massive pericardial and right pleural hematoma was evident. Hematoma evacuation and bleeding control was done, with right atrial appendage venous cannula relocation.
On day 10, the patient's condition had been improved. ECMO weaning was tried, and inotropes and vasopressor were tapered cautiously. Serologic tests for occult pheochromocytoma were negative (plasma metanephrine 0.15 nmol/L, normetanephrine 0.74 nmol/L).
On day 15 following admission, a follow-up echocardiography revealed completely recovered LV function (ejection fraction 61%) and LV regional wall motion abnormality. The patient had cardiac magnetic resonance imaging (MRI) a month after admission, which showed no evidence of delayed enhancement of LV myocardium and perfusion defect, which was compatible with SCMP (Fig. 4). Two months after admission, the patient had no back pain and was discharged. A month after discharge, follow up electrocardiography revealed normalized ST segment (Fig. 1). The patient has been free of cardiac symptoms including dyspnea, edema, and chest pain for 1 month.
SCMP is a transient and localized cardiac wall motion abnormality. It has become an increasingly reported clinical syndrome accounting for about 0.02% of all hospitalization.6) Presentation as a severe form is common, but symptoms usually resolve without invasive interventions due to the transient course.1) But, reported in-hospital mortality rates range from 0% to 8% with a 3.5% recurrence rate.7)8)9)10)
The Mayo criteria are most often used to diagnose SCMP. All of the following are required: transient wall motion abnormality of the mid-LV segments with or without apical involvement, absence of obstructive coronary disease, new electrocardiography abnormalities or cardiac troponin elevation, and absence of pheochromocytoma or myocarditis.11) Apart from these diagnostic criteria, morphological variants of stress induced cardiomyopathy have also been reported.4) SCMP is often associated with smoking, alcohol abuse, anxiety, and dyslipidemia.6) Recent study proved that younger patients with physical triggers and acute neurologic or psychiatric diseases had increased incidence of acute complications.5)
In this case, the patient's initial TTE findings showed akinesia involving the apex and all mid-LV walls, with relatively preserved contractility at all basal LV walls. An intact coronary artery was revealed on coronary angiography. Thorough serologic examination ruled out pheochromocytoma. The diagnosis was SCMP. Cardiac MRI showed functionally intact myocardium, which also suggested reversible cardiomyopathy. Late gadolinium enhancement on cardiac MRI is generally absent in SCMP, in contrast to myocardial infarction or myocarditis.12) Especially for myocarditis, it is known that there is a significantly increased relative enhancement on days 2, 7, 14 days of disease onset and no further increase is seen on day 28 and 84. Wagner et al.13) reported that the patchy areas of hyperenhancement are detectable on day 28 and 30 months after the acute onset of myocarditis on cardiac MRI. Absence of enhancement on MRI helped diagnosis of SCMP and differentiation of SCMP from myocarditis.
The patient had no known risk factors leading to coronary artery disease or reversible cardiomyopathy. Moreover, unlike most cases reported to date, the patient underwent a clinical course that would have proved fatal, which required cardiac resuscitation and ECMO to maintain life. After a month of treatment, ventricular function had returned to normal on a follow-up echocardiogram.
In conclusion, although SCMP usually has a self-resolving and benign course, it can become life-threatening requiring cardiac resuscitation and ECMO. As for other reversible cardiomyopathy, ECMO can be successfully bridge the time between ventricular dysfunction and recovery.
Figures and Tables
Fig. 1
Electrocardiographic findings revealed sinus tachycardia with ST segment elevation in precordial leads at initial admission (A) and normalized ST segment on follow up electrocardiogram at 1 month after discharge (B).
Fig. 2
Transthoracic echocardiography, parasternal long axis view (A at diastolic period, B at systolic period), and apical 4-chamber view (C at diastolic period, D at systolic period), showed dilated cavity, akinesia at all mid to apical left ventricular (LV) walls and relatively preserved contractility in basal walls, with severe LV systolic dysfunction (estimated LV ejection fraction, 15–20%) (B).
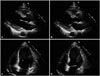
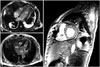
Fig. 3
Coronary angiography showing normal coronary arteries, without significant stenosis. LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery.

Fig. 4
Cardiac magnetic resonance imaging showing no evidence of LV wall motion abnormality on white blood cine image (A), myocardial perfusion defect in gadolinium-based contrast enhanced first-pass perfusion image (B). There was no evidence of delayed enhancement of LV myocardium (C). LA: left atrium, LV: left ventricle, RA: right atrium, RV: right ventricle.
References
1. Wan SH, Liang JJ. Takotsubo cardiomyopathy: etiology, diagnosis, and optimal management. Res Rep Clin Cardiol. 2014; 5:297–303.
2. Cesário V, Loureiro MJ, Pereira H. [Takotsubo cardiomyopathy in a cardiology department]. Rev Port Cardiol. 2012; 31:603–608.
3. Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008; 118:397–409.
4. Boland TA, Lee VH, Bleck TP. Stress-induced cardiomyopathy. Crit Care Med. 2015; 43:686–693.
5. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015; 373:929–938.
6. Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of takotsubo cardiomyopathy in the United States. Am Heart J. 2012; 164:66–71.e1.
7. Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008; 118:2754–2762.
8. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004; 141:858–865.
9. Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, Maron BJ. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005; 111:472–479.
10. Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I. Angina Pectoris-Myocardial Infarction Investigations in Japan. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina pectoris-myocardial infarction investigations in Japan. J Am Coll Cardiol. 2001; 38:11–18.
11. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (tako-tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008; 155:408–417.
12. Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, Thiele H, Friedrich MG. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011; 306:277–286.
13. Wagner A, Schulz-Menger J, Dietz R, Friedrich MG. Long-term follow-up of patients paragraph sign with acute myocarditis by magnetic paragraph sign resonance imaging. MAGMA. 2003; 16:17–20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download